

UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C.DC 20549
SCHEDULE 14A
PROXY STATEMENT PURSUANT TO SECTION 14(a) of the Securities Exchange Act of
OF THE SECURITIES EXCHANGE ACT OF 1934
(Amendment No. )
 | |||
Filed by the Registrant |  | Filed by a Party other than the Registrant |
| Check the appropriate box: | |
 | Preliminary Proxy Statement |
 | Confidential, for Use of the Commission Only (as permitted by Rule |
 | Definitive Proxy Statement |
 | Definitive Additional Materials |
 | Soliciting Material under §240.14a-12 |


VERTEX PHARMACEUTICALS INCORPORATED
(Name of Registrant as Specified Inin Its Charter)
(Name of Person(s) Filing Proxy Statement, if other than the Registrant)
| Payment of Filing Fee (Check | ||
 | No fee required. | |
 | Fee paid previously with preliminary materials. | |
 | Fee computed on table | |



 | A Letter from our Executive Chairman and Chief Executive Officer |
Dear Shareholders:
2023 was a transformative year for Vertex. We extended our leadership in cystic fibrosis (“CF”), expanded into new disease areas with CASGEVY regulatory approvals and commercial launches for severe sickle cell disease (“SCD”) and transfusion-dependent beta thalassemia (“TDT”) in multiple regions, and continued the rapid advancement of tremendous successour broad pipeline, which offers the potential for multiple commercial launch opportunities in disease areas outside of CF over the next few years. We believe that these important advances in 2023, combined with our continued excellent financial performance, fuel a new era of medical and commercial diversification for Vertex and more importantly,significant value creation for patients and shareholders alike.
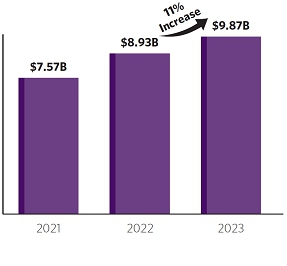
Net product revenues from our CF medicines grew to $9.87 billion in 2023, representing an 11% increase from 2022. We continued to invest significantly in innovation — both internal and external — to support our differentiated research and development (“R&D”) approach, focused on validated targets that address causal human biology, biomarkers that translate from bench to bedside, efficient development and regulatory pathways, and product candidates with transformative potential. Our differentiated R&D strategy has continued to deliver. We have obtained approvals for the thousands of people acrossfirst CRISPR/Cas9 gene-edited cell therapy in the world for the treatment of SCD and TDT and we have also delivered positive Phase 3 results for VX-548, our novel NaV1.8 pain signal inhibitor in acute pain, and for our triple combination of vanzacaftor/tezacaftor/deutivacaftor in CF. Our programs in neuropathic pain, APOL1-mediated kidney disease, and type 1 diabetes have all passed the proof-of-concept stage and represent additional multi-billion-dollar market potential. In total, our clinical-stage pipeline now spans 10 disease areas and multiple modalities, including small molecules, oligonucleotides, and cell and genetic therapies.
Along with cystic fibrosis (CF). Our dream has beendeveloping and commercializing transformative medicines for people with serious diseases, we continue to create a company that has the scientific wherewithalsupport patients, our local communities, and our employees. Last year, Vertex celebrated its 15th Global Day of Service, with participation by 60% of employees contributing nearly 8,300 hours of volunteer work across more than 125 projects with 73 non-profit groups. In addition, Vertex and the financial strengthVertex Foundation provided more than $42 million in charitable donations, with a focus on education, innovation, health, and our local communities. We remain committed to consistently discoverrecruiting, retaining, and developdeveloping highly talented employees from a diverse range of backgrounds, promoting our employees’ continued well-being and professional development, and nurturing our unique culture, which has enabled us to deliver multiple transformative medicines to patients. Our efforts continue to be recognized externally, and in 2023, Vertex was named to Fortune 100 Best Companies to Work For® 2023, Forbes Best Employers for Diversity 2023, and 2023 PEOPLE® Companies that can treatCare.
In summary, consistent execution of our R&D and corporate strategy continues to deliver strong and durable financial results, setting up significant and sustainable long-term growth for the underlying cause of serious diseases, like CF.business. We are making that dream a reality one patient at a time.
 | |
Sincerely,
Jeffrey M. Leiden, M.D., Ph.D. | |
Executive Chairman |  Reshma Kewalramani, M.D. Chief Executive Officer and President |


Wednesday, May 15, 2024
9:00 a.m. (Eastern Time)
https://meetnow.global/MQ4XFU9
Dear Shareholders:
You are invited to attend the Vertex Pharmaceuticals Incorporated’s 2016Incorporated 2024 Annual Meeting of Shareholders. At the annual meeting, shareholders will vote:
 | to elect the eleven director nominees that are set forth in the attached proxy statement to our board of directors to serve for a one-year term until the 2025 annual meeting of shareholders and until such person’s successor has been duly elected and qualified; |
 | to ratify the selection of Ernst & Young LLP as our independent registered public accounting firm for 2024; |
 | to hold an advisory vote on our 2023 named executive officer compensation; and |
 | on two proposals submitted by our shareholders, if properly presented at the meeting. |
Shareholders also will transact any other business that may properly come before the annual meeting or any adjournment or postponement of the annual meeting.
MEETING INFORMATION
PROXY MATERIALS:
We are using the “Notice and Access” method of providing proxy materials to you planvia the Internet. We are mailing to attend the annual meeting, please ensure that your shares are represented by voting, signing, dating and returning your proxy in the enclosed envelope, which requires no postage if mailed in the United States.
 | |
MEETING ACCESS:
THE ANNUAL MEETING WILL BE HELD VIRTUALLY VIA WEBCAST. A VIRTUAL ANNUAL MEETING WILL FACILITATE SHAREHOLDER ATTENDANCE AND PARTICIPATION BY ENABLING SHAREHOLDERS TO PARTICIPATE FROM ANY LOCATION AND AT NO COST. YOU WILL BE ABLE TO PARTICIPATE IN THE MEETING ONLINE, VOTE YOUR SHARES ELECTRONICALLY, AND SUBMIT YOUR QUESTIONS DURING THE MEETING BY VISITING HTTPS://MEETNOW.GLOBAL/MQ4XFU9. THERE IS NO PHYSICAL LOCATION FOR THE ANNUAL MEETING.
Shareholders will need their unique control number, which appears on the Notice of Internet Availability of Proxy Materials or proxy card (printed in the shaded bar), or within the body of the email sending the proxy statement. If you hold shares beneficially through a bank, broker or other nominee (that is, in “street name”), you must register in advance to gain access to the virtual meeting and to beneficial holders of our common stock at
To assistregister, you in reviewingwill need to obtain a legal proxy from your bank, broker or other nominee. Once you have received a legal proxy from them, you must submit a copy of this year's proposals, we calllegal proxy, along with your attentionname and email address to Computershare at legalproxy@computershare.com. Alternatively, you may mail your legal proxy to the following address: Computershare, Vertex Pharmaceuticals Incorporated Legal Proxy, P.O. Box 43001, Providence, RI 02940-3001. Requests for registration must be labeled as “Legal Proxy” and received no later than 5:00 p.m. (Eastern Time) on May 10, 2024. You will receive an email from Computershare confirming your registration and providing your control number. You will need your control number to access the virtual annual meeting, submit your questions and vote your shares electronically.
The annual meeting will begin promptly at 9:00 a.m. (Eastern Time) on May 15, 2024.
We will make a list of our shareholders of record available electronically during the annual meeting. A shareholder wishing access to the list during the annual meeting should contact our corporate secretary in advance of the meeting.
RECORD DATE:
Only Vertex shareholders of record at the close of business on March 18, 2024 are entitled to receive notice of, and vote at, the annual meeting, and, subject to applicable law, any adjournment or postponement thereof.
VOTING:
Your vote matters. Whether or not you plan to attend the annual meeting, we urge you to vote as promptly as possible by Internet, telephone or signing, dating and returning a printed proxy summary. card. If you attend the annual meeting, you may vote your shares during the annual meeting even if you previously voted your proxy. Please vote as soon as possible to ensure that your shares will be represented and counted at the annual meeting.
April 4, 2024
By Order of the Board of Directors,

Joy Liu
Corporate Secretary
IMPORTANT NOTICE REGARDING INTERNET AVAILABILITY OF PROXY MATERIALS. This is only a summary; please review thisnotice, our proxy statement, and our Annual Report on Form 10-K for the year ended December 31, 2015,2023 are first being made available to holders of record of our common stock on or 2015 Annual Report, in full.
2023 was a transformational year for the company marked by significant advances in research, development, and commercialization, across the entire company. Our strategy of investing in scientific innovation to developingcreate transformative medicines for people with serious diseases. Overdiseases, with a focus on specialty markets, is delivering. Not only did we extend our leadership in cystic fibrosis (“CF”) by reaching more people with CF than ever before, but we also diversified commercially with the last severallaunch of CASGEVY, our gene-edited cell therapy for the treatment of sickle cell disease (“SCD”) and transfusion-dependent beta thalassemia (“TDT”). In addition, we substantially progressed our broad and diverse pipeline, prepared for additional potential near-term launch opportunities in CF and acute pain, and further strengthened our financial profile.
Today, our CF medicines are collectively being used to treat nearly three-quarters of the approximately 92,000 people with CF in North America, Europe and Australia. Our CF medicines are used by people in over 60 countries, and TRIKAFTA/KAFTRIO is now reimbursed or accessible in more than 40 of those countries. As we look to the future, we expect to continue to grow our CF business through label expansions, approvals of new medicines and expanded reimbursement. We also continue to serially innovate in CF, as demonstrated by our recent announcement of positive data from three pivotal studies evaluating our next-generation triple combination of vanzacaftor/tezacaftor/deutivacaftor (the “vanzacaftor triple”). This new triple combination regimen has demonstrated the potential to provide clinical benefits over and above TRIKAFTA, and also has the advantages of a once-daily dosing regimen and a lower royalty burden. We continue to invest in serial innovation in our CFTR modulator program and have already discovered the next-generation CFTR modulators, with the goal of creating medicines that deliver even more benefit by bringing more people with CF to carrier levels of sweat chloride, which we believe is the pivotal milestone in the journey toward restoring quality and quantity of life for people living with CF. In addition, we are pursuing a nebulized messenger RNA (“mRNA”) therapy, VX-522, designed for the more than 5,000 people with CF who cannot benefit from our CFTR modulators.
In SCD and TDT, CASGEVY is now approved for people 12 years of age and older in the United States (“U.S.”), the European Union (“E.U.”), the United Kingdom (“U.K.”), the Kingdom of Saudi Arabia (“Saudi Arabia”), and the Kingdom of Bahrain (“Bahrain”). We estimate approximately 35,000 people with severe SCD and TDT could be eligible for CASGEVY in the U.S. and Europe, with additional eligible people in Saudi Arabia and Bahrain. Pursuant to our global launch strategy, we have met or exceededbeen educating physicians, patients, caregivers, payors, and policymakers about the significant disease burden of SCD and TDT and the availability of CASGEVY as a potentially curative treatment option. We are also actively engaged with treatment centers, policymakers, and payors to ensure that eligible people have broad access to this transformative therapy. Additionally, we continue to study CASGEVY in younger age groups and work on preclinical assets for myeloablative conditioning agents that would have milder side effects, which could broaden the eligible patient population.
In our goals, building onpain program, earlier this year, we announced positive results from our leadership positionPhase 3 clinical trials evaluating VX-548, a selective, peripherally-acting small molecule inhibitor of the NaV1.8 sodium channel, in the treatment of cystic fibrosis,moderate-to-severe acute pain and our plans to submit for regulatory approval in the U.S. by mid-2024. If approved, VX-548 will be the first of a new class of medicines for acute pain in over 20 years. With an estimated 80 million patients prescribed medicines for moderate-to-severe acute pain in the U.S. every year, representing over 1 billion calendar days of treatment, we believe acute pain represents a multi-billion dollar market opportunity. We also recently announced positive results from the Phase 2 clinical trial evaluating VX-548 in diabetic peripheral neuropathy (“DPN”), a type of peripheral neuropathic pain (“PNP”), and our intention to advance VX-548 into pivotal development for DPN. We also began a Phase 2 clinical trial for patients suffering from lumbosacral radiculopathy, another type of PNP. As with acute pain, our goal is to secure a broad PNP indication for VX-548. There are an estimated 10 million patients prescribed medicines for PNP, yet these medicines have significant limitations, including limited efficacy, significant side effects, and carry the risk of addiction. We believe VX-548 holds potential for a superior overall benefit-risk profile in PNP and, given the large number of people with these conditions we believe that the U.S. PNP market represents another significant, multi-billion dollar opportunity.
In addition to these potential near-term commercial opportunities, we continue to advance our clinical-stage pipeline, which includes programs that have already achieved proof-of-concept, including programs in APOL1-mediated kidney disease (“AMKD”) and type 1 diabetes (“T1D”). In addition, VX-634 and VX-638 are being evaluated in Phase 1 clinical trials in Alpha-1 Antitrypsin Deficiency (“AATD”) and VX-670, a molecule in-licensed from Entrada Therapeutics (“Entrada”), is in Phase 1/2 development for myotonic dystrophy type 1 (“DM1”), an inherited disease affecting approximately 110,000 people in the U.S. and Europe that results in the weakening and destruction of skeletal muscles over time. Recently, we initiated a Phase 1 clinical trial of VX-407, which we are developing for autosomal dominant polycystic kidney disease (“ADPKD”), the most common genetic kidney disease, and representing our tenth disease area in the clinic.
From a financial perspective, our outstanding performance in 2023 resulted in net product revenues of $9.87 billion with strong operating margins and profitability. We are well-positioned to continue to create long-term value for both patients and shareholders with a portfolio of high-value medicines for CF, SCD and TDT, potential near-term commercial launches for products in acute pain and CF, a broad and deep pipeline across ten disease areas, clinical programs with multiple therapeutic modalities that range from small molecules to genetic and cell therapies, and an excellent financial profile.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 6
Our CF medicines, TRIKAFTA/KAFTRIO, SYMDEKO/SYMKEVI, ORKAMBI, and KALYDECO, are transforming the lives of eligible people around the globe and continue to drive our financial performance.
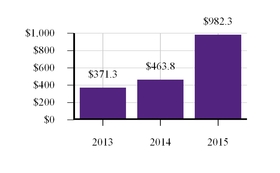
 | Our CF net product revenues increased to $9.87 billion in 2023, an increase of 11% or more than $900 million, from our 2022 CF net product revenues. |
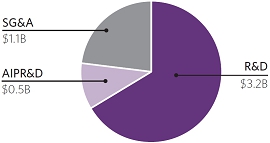
 | Our total R&D, acquired in-process research and development (“AIPR&D”) and selling, general and administrative (“SG&A”) expenses increased to $4.8 billion compared to $3.6 billion in 2022. This increase was primarily due to higher AIPR&D, increased investment in support of multiple programs that have advanced to mid- and late-stage clinical development, and the costs to support new launches of Vertex’s therapies globally. |
| INCREASING CF NET PRODUCT REVENUES | TOTAL R&D, AIPR&D AND SG&A EXPENSES |
 |  |
2023 R&D, AIPR&D AND
SG&A EXPENSES
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 7
Our differentiated R&D strategy has delivered not only a decade-long leadership position in CF, but also the historic, global approvals for CASGEVY, the first CRISPR-based gene-edited cell therapy to be approved in the world, for people with SCD or TDT.
In CF, advancingour goal is to bring highly effective treatments to all people with CF, and broadening our pipeline, increasing revenues and establishing a strong financial profile. We have two medicineswe began to do just that together arein January 2012 when KALYDECO was first approved to treat a CF population of approximately 25,000 patients1,000 people in the U.S. Since then, we have focused on growing the number of people eligible for our medicines, expanding access to our medicines in additional geographies, and seeking improved treatment options for all people with CF.
Today, our four approved CF medicines are collectively being used to treat nearly three quarters of the approximately 92,000 people with CF in North America, Europe, and Australia. Our CF medicines are used by people in over 60 countries and TRIKAFTA/KAFTRIO is now approved or approximately one thirdaccessible in more than 40 of these countries. In the near term, we expect our CF business to continue to grow as a result of (i) annualization of patients who recently initiated a CFTR modulator, (ii) label expansions, including into younger age groups and additional eligible mutations globally, (iii) expanded reimbursement, and (iv) the launch of the vanzacaftor triple, which will be a therapeutic option for those who have not yet initiated treatment with a CFTR modulator or who have discontinued from a CFTR modulator. In the mid- and longer-term, we foresee growth from (i) increases in the number of people living with CF, population worldwide. These(ii) VX-522, a potential new therapy for the treatment of the more than 5,000 people with CF who cannot benefit from CFTR modulators, and (iii) next-generation CFTR modulator regimens.
In SCD and TDT, 2023 marked the commercial launch of CASGEVY, following approvals in the U.S., U.K., and Bahrain, and with approvals in the E.U. and Saudi Arabia following closely in early 2024. We estimate approximately 35,000 people with severe SCD or TDT could be eligible for CASGEVY in the U.S. and Europe, with additional eligible people in Saudi Arabia and Bahrain. Our global launch strategy for CASGEVY is focused on disease education and awareness for patients, caregivers, health care professionals, payors, and policymakers, as well as engagement with the scientific and medical community regarding CASGEVY clinical data. Our strategy is also focused on activating authorized treatment centers to ensure their readiness to treat patients and achieving access for patients through reimbursement agreements with governments and commercial payors, as well as through early access programs where applicable.
Since the beginning of 2023, notable progress includes:
 | The U.S. Food and Drug Administration (“FDA”), the European Commission, the U.K. Medicines and Healthcare products Regulatory Agency (“MHRA”), and Health Canada approved TRIKAFTA/KAFTRIO for the treatment of children with CF 2 to 5 years of age who have at least one F508del mutation in the CFTR gene. |
 | The FDA and the European Commission approved the use of ORKAMBI for children with CF from 12 months to less than 24 months of age who are homozygous for the F508del mutation. |
 | The FDA approved KALYDECO in children with CF from 1 month to less than 4 months of age. |
 | The approval of CASGEVY in the U.S., E.U., U.K., Saudi Arabia, and Bahrain for people 12 years of age and older with SCD or TDT. |
 | We have engaged with the Medicaid administrators in all 50 U.S. states, focused on the 25 states with the highest prevalence of SCD patients, and have confirmed pathways to reimbursement in nearly all 25 of these priority states. |
 | Approval by the French National Authority for Health of our request for the implementation of an early access program for the use of CASGEVY to treat eligible people with TDT from 12 to 35 years of age. |
 | We have begun engagement with payors in the U.K., E.U., Saudi Arabia and Bahrain, including engagement with the National Institute for Health and Care Excellence. |
 | Submission for approval of CASGEVY in both SCD and TDT in Switzerland. |
 | Activation of 16 authorized treatment centers in the U.S., four authorized treatment centers in Europe, and one in Saudi Arabia. |
 | Entry into an agreement with Synergie Medication Collective, a medication contracting organization, covering approximately 100 million people in the U.S., to provide access to CASGEVY. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 8
We are preparing for the following potential near-term launches of two new products:
 | Vanzacaftor triple in CF. We continued our strategy of serial innovation by completing three pivotal studies evaluating our once-daily triple combination CFTR modulator therapy, vanzacaftor/tezacaftor/deutivacaftor. Results of the clinical trials demonstrate that this triple combination has the potential to provide additional clinical benefits beyond TRIKAFTA for people with CF who have at least one mutation in their CFTR gene responsive to CFTR modulators. This regimen also has the advantages of once-daily dosing and a lower royalty burden compared with TRIKAFTA. We expect to support the launch of the vanzacaftor triple with our existing commercial infrastructure. We expect to submit global regulatory filings for this triple combination by mid-2024, including in the U.S., the E.U., and Canada for people with CF 6 years and older. In the U.S., we will be using one of our priority review vouchers to shorten the regulatory review period from ten months to six months. |
 | VX-548 in acute pain. We completed three Phase 3 clinical trials for VX-548, a non-opioid, investigational selective NaV1.8 inhibitor, for the treatment of moderate-to-severe acute pain. Results of the clinical trials indicate that VX-548 could provide a transformative option for patients suffering from acute pain, based on the suboptimal benefit risk profile of existing agents, including the adverse effects and addictive potential of opioids, and the favorable benefit risk profile of VX-548. For our potential near-term commercial opportunity in acute pain, we are focused on the multi-billion dollar market arising from the estimated 80 million patients in the U.S. who are prescribed a medicine for their moderate-to-severe acute pain each year. More than two-thirds of patients receive acute pain prescriptions either during a hospital or ambulatory surgery center visit or at discharge; these prescriptions are concentrated in approximately 2,000 hospitals and 200 integrated delivery networks, which we believe we can reach with a specialty sales force. We plan to submit a New Drug Application (“NDA”) for the treatment of moderate-to-severe acute pain to the FDA by mid-2024. |
We invest in research and development to discover and develop transformative medicines arefor people with serious diseases, with a focus on specialty markets. Our research and development strategy combines advances in the first,understanding of human disease and only,the science of therapeutics to dramatically advance human health. This strategy was designed to deliver transformative medicines that treatfor serious diseases at high rates of speed and success, and it has delivered just that. Our success in moving novel product candidates into clinical trials, successfully completing pivotal development and obtaining marketing approvals offer multiple proof points of this strategy, and include TRIKAFTA/KAFTRIO, SYMDEKO/SYMKEVI, ORKAMBI, and KALYDECO for CF, and CASGEVY for SCD and TDT. The strategy continues to be borne out by our pipeline, which includes potential future approvals of the underlying causevanzacaftor triple for the treatment of CF and we believe theyVX-548 for the treatment of acute pain. Our approach to drug discovery has also yielded therapies that have fundamentally changeddemonstrated clinical proof-of-concept in additional disease areas, including neuropathic pain with VX-548, AMKD with inaxaplin, and T1D with VX-880, a stem cell-derived islet cell therapy.
Our research and development approach also includes pursuing multiple modalities tailored to the way eligible patients can be treated.specific disease area target under investigation, using biomarkers that translate from the bench to the bedside, and advancing multiple candidates into clinical trials with the goal of bringing first-in-class, followed by best-in-class, therapies to patients. In addition to expanding our small molecule programs, we have a strong CF pipeline, withalso advanced an industry-leading portfolio of programs in cell and genetic therapies.
Our advancements across multiple drug candidates, that may allow us to help all patients with this raredisease areas and life-shortening disease.
 | Cystic Fibrosis. We continue to pursue next-in-class, small molecule CFTR modulator therapies and have already identified next-in-class correctors and potentiators, as well as genetic therapies for people with CF who do not make full-length CFTR protein and, as a result, cannot benefit from our current CF medicines. For these more than 5,000 people with CF, in collaboration with Moderna, we are developing VX-522, a CF mRNA therapeutic designed to treat the underlying cause of CF in these people by enabling cells in the lungs to produce functional CFTR protein. We have completed dosing in the single ascending dose portion of the clinical trial for VX-522 in people with CF and initiated the multiple ascending dose portion of the trial. |
 |
| eligible patient population. In addition, we are investigating small molecules for the potential treatment of SCD and TDT. | |
 |
| moderate-to-severe acute pain in 2024. We also anticipate initiating a Phase 1 study of an intravenous formulation of VX-993 in 2024. | |
 |
| this year. We |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 9
 | APOL-1 Mediated Kidney Disease. We completed enrollment in the Phase 2B dose-ranging portion of the clinical trial evaluating inaxaplin for the treatment of AMKD and have selected the dose for and initiated the Phase 3 portion of the Phase 2/3 pivotal clinical trial. |
 | Type 1 Diabetes. We completed Parts A and B, and completed enrollment in Part C of the Phase 1/2 clinical trial evaluating VX-880, an allogeneic, stem-cell derived, fully-differentiated, insulin-producing islet cell therapy, used in conjunction with standard immunosuppression, for the treatment of T1D in people with impaired awareness of hypoglycemia and recurrent hypoglycemic events. We have placed the study on a protocol-specified pause, pending review of the totality of the data by the independent data monitoring committee. The clinical trial for our second program in T1D, VX-264, in which the allogeneic stem-cell derived, fully-differentiated, insulin-producing islet cells are encapsulated and implanted in an immunoprotective device to obviate the need for immunosuppression, is a multi-part Phase 1/2 study. We have completed enrollment and dosing in Part A, and Part B of the clinical trial is underway in multiple centers and countries. |
 | Myotonic dystrophy type 1. We are exploring multiple approaches to address the underlying causal biology for DM1, including an oligonucleotide linked to a circular peptide, VX-670, which was in-licensed from Entrada. The Investigational New Drug Application (“IND”) for the Phase 1/2 clinical trial of VX-670 in people with DM1 has cleared, as have the regulatory submissions in Canada, the U.K. and multiple other geographies. The study has been initiated in Canada and is expected to initiate in other regions in the near term. |
 | Alpha-1 Antitrypsin Deficiency. We continue to enroll and dose Phase 1 clinical trials evaluating VX-634 and VX-668. |
 | Autosomal Dominant Polycystic Kidney Disease. We completed pre-clinical enabling studies for VX-407, our first-in-class small molecule corrector that targets the underlying cause of ADPKD in people with a subset of PKD1 genetic variations, in late 2023. The IND for VX-407 in the U.S. has cleared and we have initiated a Phase 1 clinical trial evaluating VX-407 in healthy volunteers in the U.S. |
 | In addition to the programs listed above, we have several earlier-stage research programs aimed at diseases that fit our R&D strategy, as well as follow-on programs in diseases already in the clinic. |
We will continue investing in our research and development programs and fostering scientific innovation by identifying additional product candidates through our internal research efforts and investing in business development transactions to access emerging technologies, products and product candidates.
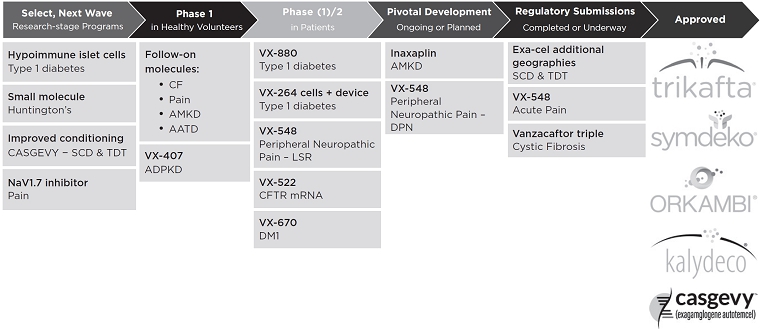
The following chart represents our clinical stage programs and select pre-clinical programs.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 10
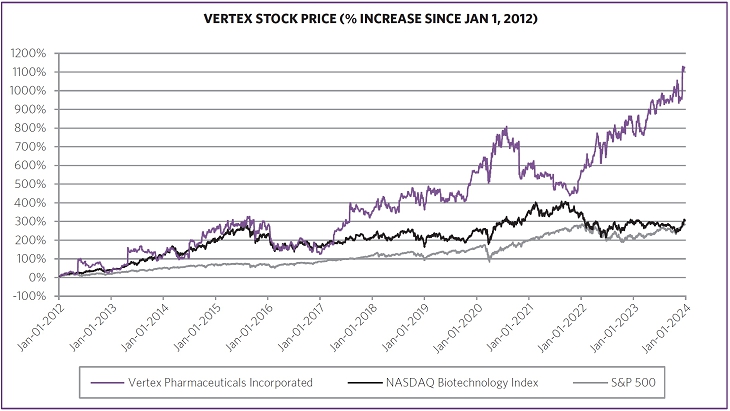
Driven by our financial strengthperformance and became cash flowpipeline successes, our stock price increased 40.9% from $288.78 per share at the end of 2022 to $406.89 per share at the end of 2023. We believe biotechnology companies are best measured over the long term, as opposed to one-year or other shorter-term increments. The following charts show our total shareholder return relative to the Nasdaq Biotechnology Index (“NBI”) and S&P 500 index since the beginning of 2012, when our first CF medicine was approved, as well as our stock price performance over multiple periods. We believe the execution of our differentiated research and development approach and corporate strategy will continue to create shareholder value over the long term.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 11
We are a leading global biotechnology company that serially innovates to bring transformative medicines to people with serious diseases. We are committed to operating our business responsibly and disclosing our progress to stakeholders on an annual basis. Our progress and efforts with respect to environmental, social, and governance topics, including community engagement and workplace practices, were recognized broadly in 2023. A selection of awards and recognitions include Fortune 100 Best Companies to Work For® 2023, Forbes’ Best Employers for Diversity, PEOPLE® Magazine’s 100 Companies That Care, Points of Light’s Civic 50, Science Magazine’s Top Employers, Seramount’s 100 Best Companies, and many others.
Our corporate responsibility priorities relate to four objectives fundamental to our business: improving the lives of people with serious diseases; fostering a culture of innovation, integrity, and inclusion; carefully managing our operations and environmental footprint; and making a positive impact in the fourth quarter of 2015, allowing us to continue to invest significantly in R&D and return value to shareholders:
 | Improve the lives of people with serious diseases | We are focused on discovering, developing and producing innovative medicines so that people with serious diseases can lead better lives. We invest significantly in research and development, with the majority of operating expenses and our workforce dedicated to that purpose. Once we discover transformative medicines, we then work to ensure patients have access to our medicines. We are deeply committed to understanding the challenges and unmet needs of patients and recognize the importance of partnering with, elevating, and empowering patient communities. |
 | We are focused on fostering a culture of innovation, integrity and inclusion. Our culture of high ethical standards and integrity is one of the key components to |
| our work every day. We are committed to building an outstanding, committed and passionate team, and we value inclusion, diversity and equity to foster creativity and innovation. Five of our eleven director nominees, including our chief executive officer (“CEO”), are women, and four of our eleven director nominees are from underrepresented communities. As of December 31, 2023, women represented 55% of our global workforce and 40% of our global leadership (vice president and above). In the U.S., in the year ending December 31, 2023, employees from underrepresented ethnic and racial groups represented approximately 41% of our workforce and 45% of new hires. To promote our employees’ continued well-being and development, we also offer a variety of inclusive benefits and career development opportunities. | ||
 | We are committed to limiting our environmental impact and to operating our business in a sustainable manner. In 2023, we established a new target to reduce our Scope 1 and Scope 2 absolute greenhouse gas (“GHG”) emissions. 49% of our global energy comes from renewable energy sources and we source 100% renewable energy for our U.K.-based international headquarters and R&D facility. For our continued efforts, we received a score of an A- on the 2023 CDP Climate Change survey, demonstrating environmental leadership (global average score is C). We also continually improve standards and incorporate industry best practices with | |
 | Make a positive impact in | We continue to support communities through collaborations, donations, and |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 3
The following table provides summary information regarding our eleven director nominees. For detailed information about each nominee’s background and areas of expertise, please see Proposal No. 1: Election of Directors.
| Committees | ||||||||||||||
| Name, Occupation or Experience | Age | Director Since | Independent | AC | MDCC | CGNC | S&T | |||||||
Jeffrey Leiden Executive Chairman, Vertex | 68 | 2009 | No | |||||||||||
Reshma Kewalramani CEO and President, Vertex | 51 | 2020 | No | |||||||||||
Sangeeta Bhatia John J. and Dorothy Wilson Professor of Health Sciences & Technology/Electrical Engineering & Computer Science, MIT | 55 | 2015 | Yes |  |  | |||||||||
Lloyd Carney Former CEO, Brocade Communications | 62 | 2019 | Yes |  |  | |||||||||
Alan Garber Interim President and Provost, Harvard University | 68 | 2017 | Yes |  |  | |||||||||
Michel Lagarde Executive Vice President and Chief Operating Officer, Thermo Fisher Scientific Inc | 50 | 2023 | Yes |  | ||||||||||
Diana McKenzie Former Chief Information Officer, Workday | 59 | 2020 | Yes |  |  |  | ||||||||
Bruce Sachs Partner Emeritas, Charles River Ventures | 64 | 1998 | Yes |  |  | |||||||||
Jennifer Schneider Co-Founder and CEO, Homeward Health, Inc. | 49 | Nominee | Yes | |||||||||||
Nancy Thornberry Former CEO, Kallyope, Inc. | 67 | 2023 | Yes |  | ||||||||||
Suketu Upadhyay Executive Vice President and Chief Financial Officer, Zimmer Biomet | 55 | 2022 | Yes |  | ||||||||||
 = Chair = Chair | ||||||||||||||
VERTEX PHARMACEUTICALS INCORPORATED- 2024 Proxy Statement 13
In 2023, our executive compensation program and in particular, toreceived substantial support, with approval by approximately 89% of the equity compensation component, which were implemented in early 2016. The new equity compensation reflects fundamental changes in our business and financial profile and feedback we received from our shareholders. While we continually engage in dialoguevotes cast at the annual meeting. We believe this support is consistent with our shareholders, we increased our level of engagement in response to the decline in support for our advisory say-on-pay proposal at our 2015 annual meeting. Over the past year, we held specific discussions regarding executive compensation with shareholders representing approximately 75% of our outstanding stock.
 | We maintained the base salary and target equity level for Dr. Reshma Kewalramani, our CEO and President, based on a comparative analysis of companies in our peer group, and maintained her target cash bonus as a percentage of base salary. |
 | We maintained target compensation for Mr. Stuart Arbuckle, Dr. David Altshuler, and Mr. Charles Wagner, based on a comparative analysis of companies in our peer group. |
 | Dr. Leiden continues to receive no cash compensation for his role as Executive Chairman other than an annual cash payment intended to facilitate participation in the company’s benefit plans, and he will continue to receive equity awards for his fifth year of service as Executive Chairman. |
 | Our outstanding performance in 2023 resulted in the board determining that we had achieved a leading rating for 2023 (150 out of a potential 150), with the payment of annual cash bonuses commensurate with this high level of performance. |
 | We maintained the mix of equity granted to our NEOs with 50% of the awards consisting of performance stock units (“PSUs”) that vest upon achievement of specific performance goals and 50% consisting of time-vesting restricted stock units (“RSUs”). This mix rewards stock price appreciation and incentivizes long-term tenure. |
We believe that a proxy access frameworkrobust shareholder outreach program is an important component of maintaining our strong corporate governance practices. We strive for a collaborative approach with shareholders to solicit and understand a variety of perspectives and interests, and our practice has been to engage with our shareholders regularly over the course of the year.
During 2023, we solicited feedback regarding our corporate governance practices from our top 40 shareholders representing approximately 65% of our outstanding shares. Our integrated outreach team included leaders from our Investor Relations, Human Resources, Corporate Responsibility, Corporate Communications, and Legal teams, and we discussed numerous topics of shareholder interest, including our business strategy, R&D approach, diversity initiatives and metrics, employee engagement and development, corporate governance, political and lobbying disclosures, executive compensation, and environmental sustainability matters.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 14
We are committed to maintaining strong corporate governance practices that it believes will provide meaningful access for shareholders while safeguardingpromote the interestlong-term interests of all our shareholders and limiting the potential for abuse.
| Item 1: | FOR |
| Item 2: | FOR |
| Ratify Selection of Independent Auditor for | |
| Item 3: | FOR |
| Approve, on an Advisory Basis, Our Named Executive Officer Compensation | |
| Item | AGAINST |
| Item | AGAINST |
| Shareholder Proposal Regarding |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 15
Table of Annual Meeting of Shareholders
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 5
| Frequently Asked Questions Regarding the Annual | Item 6: Shareholder Proposal #3 | 34 | ||
| Meeting | 7 | Item 7: Shareholder Proposal #4 | 36 | |
| Item 1: Election of Directors | 10 | Compensation Discussion and Analysis | ||
| Board Structure and Composition | 10 | Overview | 38 | |
| Shareholder-Recommended Director Candidates | 11 | Detailed Discussion and Analysis | 46 | |
| Proxy Access By-law | 11 | Management Development and Compensation | ||
| Majority Vote Standard | 12 | Committee Report | 66 | |
| Director Nominees | 13 | Compensation and Equity Tables | 67 | |
| Continuing Directors | 15 | Summary Compensation Table | 67 | |
| Corporate Governance and Risk Management | 18 | Option Exercises and Stock Vested for 2015 | 68 | |
| Independence, Chair and Co-Lead Independent | Total Realized Compensation Table | 69 | ||
| Directors | 18 | Grants of Plan-Based Awards During 2015 | 70 | |
| Board Committees | 18 | Outstanding Equity Awards at Fiscal Year-End | ||
| Risk Management | 19 | for 2015 | 72 | |
| Code of Conduct | 19 | Summary of Termination and Change of Control | ||
| Board Attendance, Committee Meetings and | Benefits | 75 | ||
| Committee Membership | 20 | Employment Contracts and Change of Control | ||
| Audit and Finance Committee | 20 | Arrangements | 76 | |
| Corporate Governance and Nominating | Equity Compensation Plan Information | 85 | ||
| Committee | 20 | Security Ownership of Certain Beneficial | ||
| Management Development and Compensation | Owners and Management | 86 | ||
| Committee | 21 | Section 16(a) Beneficial Ownership Reporting | ||
| Compensation Committee Interlocks and | Compliance | 87 | ||
| Insider Participation | 21 | Other Information | 88 | |
| Science and Technology Committee | 21 | Other Matters | 88 | |
| Director Compensation | 22 | Shareholder Proposals for the 2017 Annual | ||
| Item 2: Ratification of the Appointment of | Meeting and Nominations for Director | 88 | ||
| Independent Registered Public Accounting Firm | 25 | Shareholder Communications to the Board | 88 | |
| Audit and Finance Committee Report | 27 | Householding of Annual Meeting Materials | 88 | |
| Item 3: Advisory Vote to Approve Named | Solicitation | 89 | ||
| Executive Officer Compensation | 28 | Availability of Materials | 89 | |
| Item 4: Shareholder Proposal #1 | 30 | Forward Looking Statements | 89 | |
| Item 5: Shareholder Proposal #2 | 32 | |||
This proxy statement with the enclosed proxy card, is being furnishedmade available to shareholders of Vertex Pharmaceuticals Incorporated in connection with the solicitation by our board of directors of proxies to be voted at our
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 6
| Back of Contents |
Our board of directors has nominated Joshua Boger, Terrence C. Kearney, Yuchun Leethe following current directors - Sangeeta Bhatia, Lloyd Carney, Alan Garber, Reshma Kewalramani, Michel Lagarde, Jeffrey Leiden, Diana McKenzie, Bruce Sachs, Nancy Thornberry, and Elaine S. UllianSuketu Upadhyay - as well as Jennifer Schneider, for re-electionelection at our 20162024 annual meeting of shareholders to hold office until our 20192025 annual meeting of shareholders.
Each of the nominees has agreed to be named in this proxy statement and to serve if elected. We believe that all of the nominees will be able and willing to serve if elected. However, if any nominee should become unable for any reason or unwilling to serve for any reason, proxies may be voted for another person nominated as a substitute by our board or our board may reduce the number of directors.
Our board of directors is our company’s ultimate decision-making body, except with respect to those matters reserved to the shareholders. Our board selects our senior management team, which in turn is responsible for the day-to-day operations of our company. Our board acts as an advisor and counselor to senior management and oversees its performance.
The corporate governance and nominating committee (“CGNC”) of our board of directors is responsible for recommending the composition and structure of our board, including identifying, developing, and for developing criteriarecommending qualified candidates for board membership. This committeeThe CGNC regularly reviews director competencies, qualities, skills, and experiences with the goal of ensuring that our board is comprised of an effective teamconsists of directors who function collegially and effectively and who are able to apply their experience toward meaningful contributions to general corporate strategy and oversight of corporate performance, risk management, organizational development, and succession planning.
Our by-laws provide that the size of our board may range between three and eleven members. We currently have nineeleven members on our board. Following our 2016 annual meetingboard and the election of the four directors, we expect that Dr. Boger will resign as a Class III director and will be re-appointed to our board as a Class I Director, with a term expiring in 2017, in order to ensure that the number ofhave eleven members of each class of our board immediately following the 2024 annual meeting of directors remains as nearly equal as possible.shareholders. Our corporate governance and nominating committeeCGNC may seek additional director candidates in the future who meet the criteria below in order to complement the qualifications and experience of our existing board members. Our corporate governance and nominating committeeCGNC may engage a search firm to recommend candidates who satisfy thesuch criteria.
The corporate governance and nominating committeeCGNC seeks to recommend for nomination experienced directors of stature who have a substantive knowledge of our business and industry or who can bring to the board specific and valuable strategic or management capabilities acquired in other industries. The committee expects each of our directors to have proven leadership, sound judgment, the highest ethics and integrity, and a commitment to the success of our company. Wesuccess. It also seekseeks personal qualities that foster a respectful environment in which our directors listen to one another and are engagedengage in robust and constructive.constructive discussions. These goals for our board composition presuppose a diverse range of viewpoints, experiences, and specific expertise. The corporate governance and nominating committeeCGNC considers a nominee’s personal characteristics and business experience relative to those of our existing board members, including the type of prior management experience, levels of expertise relevant to our business, and its growth stage, prior board service, reputation in the business community, personal characteristics such as gender and race, and other factors that the committee believes to be important. At this time,When considering whether or not to re-nominate a director for board service, the CGNC also considers whether the director has served as a member of our board for more than 20 years and whether the director is over 72 years of age. Jennifer Schneider was selected and recommended as a nominee for our board of directors by the CGNC based on the criteria outlined above.
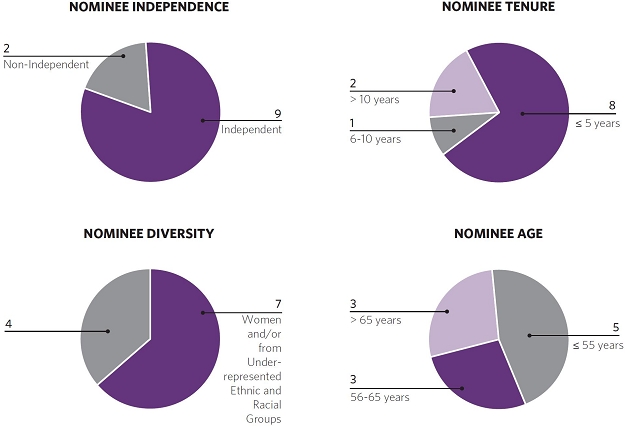
Our commitment to diversity and inclusion is demonstrated by the composition of our board nominees, which includes threefive women and two ethnically diverse individuals.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 18
The key experience, qualifications, attributesfollowing table and skills broughtcharts provide information regarding our director nominees:
 |  |  |  |  |  |  |  |  |  |  | |
| Leadership Experience. We believe that directors who have held significant leadership positions over extended periods of time provide our company with special insights. |  |  |  |  |  |  |  |  |  |  |  |
| Industry Knowledge. We seek directors with substantive knowledge of the healthcare and biotechnology industries to successfully advise and oversee the strategic development and direction of our company. |  |  |  |  |  |  |  |  |  | ||
| Financial Expertise. We believe that an understanding of finance is important for members of our board, and our budgeting processes and financial and strategic transactions require our directors to be financially knowledgeable. |  |  |  |  |  |  |  |  |  |  | |
| International Perspective. We have significant operations outside the United States and value directors with experience in the operation of complex multinational organizations. |  |  |  |  |  |  |  |  | |||
| Public Policy and Regulation. We operate in a highly-regulated industry and seek directors who have experience in public policy and the regulation of medicines. |  |  |  |  | |||||||
| Academic Experience or Technological Background. As a biotechnology company that seeks to develop transformative medicines for patients with serious diseases, we look for directors with backgrounds in academia, science and technology and, in particular, the research and development of pharmaceutical products. |  |  |  |  |  |  |  |  |  | ||
| Commitment to Company Values and Goals. We seek directors who are committed to our company and its values and goals and who value the contributions that can be provided by individuals who believe in our company and its prospects for success. |  |  |  |  |  |  |  |  |  |  |  |
| Independence | Y | Y | Y | N | Y | N | Y | Y | Y | Y | Y |
| Age | 55 | 62 | 68 | 51 | 50 | 68 | 59 | 64 | 49 | 67 | 55 |
| Tenure on Board | 8 | 5 | 6 | 4 | 0* | 14 | 4 | 25 | 0 | 0** | 2 |
| Gender | F | M | M | F | M | M | F | M | F | F | M |
| Underrepresented Ethnic and Racial Groups |  |  |  |  |
| * | Mr. Lagarde was appointed to the Board on October 5, 2023. |
| ** | Ms. Thornberry was appointed to the Board on December 5, 2023. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 19
Our commitment to diversity and inclusion is also demonstrated by our directors to our board that are important to our business include:
| Board Diversity Matrix as of April 4, 2024 | ||||||||||
| Total Number of Directors | 11 | |||||||||
| Female | Male | Non-Binary | Did Not Disclose Gender | |||||||
| Part I: Gender Identity | ||||||||||
| Directors | 4 | 7 | 0 | 0 | ||||||
| Part II: Demographic Background | ||||||||||
| African American or Black | 0 | 1 | 0 | 0 | ||||||
| Alaskan Native or Native American | 0 | 0 | 0 | 0 | ||||||
| Asian | 2 | 1 | 0 | 0 | ||||||
| Hispanic or Latinx | 0 | 0 | 0 | 0 | ||||||
| Native Hawaiian or Pacific Islander | 0 | 0 | 0 | 0 | ||||||
| White | 2 | 5 | 0 | 0 | ||||||
| Two or More Races or Ethnicities | 0 | 0 | 0 | 0 | ||||||
| LGBTQ | 0 | |||||||||
| Did Not Disclose Demographic Background | 0 | |||||||||
The corporate governance and nominating committeeCGNC will consider director candidates recommended by shareholders using the same criteria for director selection described above under
Our by-laws and adoptedprovide for proxy access, a process that allows qualifying shareholders to nominate a director candidate for consideration at an annual meeting of shareholders and have such candidate be included in our proxy materials for the applicable shareholder meeting. This amendment was in response to the approval of a shareholder proposal at our 2015 annual meeting. Our engagement with our shareholders regarding proxy access included discussions over the last several months with a number of our largest shareholders that together hold approximately 46% of our outstanding stock. This engagement allowed us to gain valuable feedback as to the particular proxy access parameters that our shareholders consider appropriate. Based on that feedback and after considering various proxy access provisions recently adopted by other companies, including biotech peers such as Alexion and pharmaceutical companies such as Pfizer, our board of directors adopted a proxy access framework that it believes will provide meaningful access for shareholders while safeguarding the interest of all our shareholders and limiting the potential for abuse. The key elements of our proxy access by-law are as follows:
| Provision | |
| Ownership Threshold and Holding Period | Available to shareholders owning 3% or more of our voting shares continuously for at least 3 years. |
| Number of Board | Total number of proxy access nominees is capped at the greater of 20% of the existing number of board seats (or the closest whole number below 20%) |
| Aggregation Limits | 20-shareholder limit on the number of shareholders who can aggregate their shares to satisfy the 3% ownership requirement. |
| Proxy Fights | Proxy access nominees will not be included in the proxy materials if we receive notice that a shareholder intends to nominate a candidate who is not to be included in our proxy materials. |
| Future Ineligibility | Proxy access nominees who fail to receive at least 10% of the votes cast |
The above table is only a summary of our proxy access by-law and is qualified in its entirety by the actual amendment to our by-laws, which is set forth in Exhibit 3.1 of a Current Report on Form 8-K, that we filed with the SEC on April 27, 2016.by-laws. A shareholder who wishes to nominate a proxy access nominee to be considered for election as a director at the
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 20
| Back of Contents |
Our by-laws provide for a majority votevotes cast standard for uncontested elections of our directors. Under our by-laws, director nominees in an uncontested election who receive more votes cast “for” such director nominee than “withheld” from“against” such director nominee are elected. Our board’s policy is that any nominee for director in an uncontested election who receives a greater number of votes “withheld”“against” than votes “for” the nominee’s election shall promptly tender his or hertheir resignation to the chair of our board following certification of the shareholder vote. Our corporate governance and nominating committeeThe CGNC will promptly consider the tendered resignation. Based on all factors it deems in its discretion to be relevant, the committeeCGNC will recommend that our board either accept or reject the resignation and may recommend that the board adopt measures designed to address any issues perceived to underlie the election results. Our board will then act on the corporate governance and nominating committee’sCGNC’s recommendation. We will promptly disclose our board’s decision, including, if applicable, the reasons for rejecting the tendered resignation. Any director whose resignation is being considered under this policy will not participate in the corporate governance and nominating committeeCGNC or board considerations, recommendations or actions with respect to the tendered resignation.
Other Public Company Boards:  None | Sangeeta Bhatia | ||
Age: 55 Director Since: 2015 | Board Committees:  Chair – Science and Technology Committee  Member – Corporate Governance and Nominating Committee | ||
Experience:  Professor at the Massachusetts Institute of Technology (“MIT”); John J. and Dorothy Wilson Professor of Health Sciences & Technology/Electrical Engineering & Computer Science, since 2005  Co-Founder of Ropirio Therapeutics, a private biotechnology company focused on lymphatic medicine since 2023  Co-Founder of Amplifyer Bio, a private biotechnology company focused on oncology diagnostics, since 2023  Co-Founder of Matrisome Bio, a private biotechnology company, since 2023  Co-Founder of Port Therapeutics, a private company focused on thermal bioswitches in oncology, since 2022  Co-Founder of Satellite Bio, a private company focused on developing satellite organs as living therapeutic solutions, since 2020  Co-Founder of Glympse Bio, a private company focused on developing in vivo sensing technology dedicated to disease monitoring, from 2018 until it was acquired by Sunbird Bio, Inc. in August 2023  Professor of bioengineering and medicine at the University of California at San Diego, from 1998 through 2005  Investigator for the Howard Hughes Medical Institute, a member of the Department of Medicine at Brigham and Women’s Hospital, a member of the Broad Institute and a member of the Koch Institute for Integrative Cancer Research  Holds a Sc.B. in biomedical engineering from Brown University, an S.M. and Ph.D. in Mechanical Engineering from MIT, and an M.D. from Harvard Medical School | |||
Key Skills and Qualifications: Dr. Bhatia is a leading academic scientist and medical researcher. Her extensive experience in the field of biomedical engineering and in-depth understanding on the use of advanced technologies in medical research provide valuable insights to our board of directors, including with respect to our key research and development initiatives. | |||
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 21
Other Public Company Boards:  Visa Inc.  Grid Dynamics Holdings Inc. | Lloyd Carney | ||
Age: 62 Director Since: 2019 | Board Committees:  Chair – Corporate Governance and Nominating Committee  Member – Management Development and Compensation Committee | ||
Experience:  Chief Acquisition Officer of Carney Technology Acquisition Corp. II, a special purpose acquisition corporation, from October 2020 until February 2023  Chief Executive Officer of ChaSerg Technology Acquisition Corp., a technology acquisition company, from September 2018 until March 2020  Chief Executive Officer and Director of Brocade Communications Systems Inc., a global supplier of networking hardware and software, from 2013 until it was acquired by Broadcom in 2017  Chief Executive Officer of Xsigo Systems, a cloud-based infrastructure solutions provider, until it was acquired by Oracle in 2012  Chief Executive Officer and Chairman of Micromuse Inc., a software solutions provider for business and service assurance, from 2003 until it was acquired by IBM in 2006  Previously held senior leadership roles at Juniper Networks, Inc., Nortel Networks Inc., and Bay Networks, Inc.  Member of the board of directors of Nuance Communications Inc., a publicly traded AI-enabled communication company, until it was acquired by Microsoft Corp in March 2022  Ambassador/Special Investment Envoy for Technology Jamaica, since May 2023  Chancellor, University of Technology, Jamaica, a public university in Jamaica, since August 2022  Holds a Bachelor of Science degree in Electrical Engineering Technology from Wentworth Institute of Technology, a Master of Science degree in Applied Business Management from Lesley College, a Honorary Doctorate degree in Engineering from Wentworth Institute of Technology and a Honorary Doctorate degree in Technology from University of Technology Jamaica | |||
Key Skills and Qualifications: Mr. Carney brings strong business judgment, honed through his time as a senior executive and board member of multiple global technology companies, to our board of directors. Mr. Carney has extensive corporate leadership experience, including service as the chief executive officer of several technology companies, as well as financial expertise. | |||
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 12
Other Public Company Boards:  Exelixis, Inc. | Alan Garber | ||
Age: 68 Director Since: 2017 | Board Committees:  Member – Audit and Finance Committee  Member – Science and Technology Committee | ||
Experience:  Interim President of Harvard University since January 2024; Provost of Harvard University and the Mallinckrodt Professor of Health Care Policy at Harvard Medical School, a Professor of Economics in the Faculty of Arts and Sciences, Professor of Public Policy in the Harvard Kennedy School of Government, and Professor in the Department of Health Policy and Management in the Harvard T.H. Chan School of Public Health, since 2011  Henry J. Kaiser Jr. Professor, a Professor of Medicine, and a Professor (by courtesy) of Economics, Health Research and Policy, and of Economics in the Graduate School of Business at Stanford University, from 1998 until 2011  Member of the National Academy of Medicine, the American Society of Clinical Investigation, the Association of American Physicians, and the American Academy of Arts and Sciences  Fellow of the American Association for the Advancement of Science, the American College of Physicians, and the Royal College of Physicians  Current Research Associate with the National Bureau of Economic Research and served for nineteen years as founding Director of its Health Care Program  Previously served as a member of the National Advisory Council on Aging at the National Institutes of Health, as a member of the Board of Health Advisers of the Congressional Budget Office, and as Chair of the Medicare Evidence Development and Coverage Advisory Committee at the Centers for Medicare and Medicaid Services  Holds an A.B. summa cum laude, an A.M. and a Ph.D., all in Economics, from Harvard University, and an M.D. with research honors from Stanford University | |||
Key Skills and Qualifications: Dr. Garber brings extensive leadership experience and knowledge regarding science, medicine, and the healthcare industry and in particular healthcare economics to our board of directors. His expertise in health care policy and as an advisor to government agencies provides our board important insights and perspectives on the issues facing our company. | |||
Other Public Company Boards:  Ginkgo Bioworks Holdings, Inc. | Reshma Kewalramani | ||
Age: 51 Director Since: 2020 | Position:  Chief Executive Officer and President | ||
Experience:  Chief Executive Officer and President of Vertex Pharmaceuticals Incorporated since April 2020  Executive Vice President and Chief Medical Officer of Vertex from 2018 through March 2020  Senior Vice President, Late Development of Vertex from 2017 until 2018  Served in roles of increasing responsibility at Amgen Inc. from 2004 to 2017, most recently as Vice President and Head of U.S. Medical Organization  Industry representative to the FDA’s Endocrine and Metabolic Drug Advisory Committee from 2014 through 2019  Holds a B.A. from Boston University and an M.D. from Boston University School of Medicine; Dr. Kewalramani completed her internship and residency in Internal Medicine at the Massachusetts General Hospital and her fellowship in Nephrology at the Massachusetts General Hospital and Brigham and Women’s Hospital combined program  Dr. Kewalramani also completed the General Management Program at Harvard Business School and is an alumna of the school | |||
Key Skills and Qualifications: Dr. Kewalramani possesses strong leadership qualities, significant experience overseeing and scaling the operations of a global enterprise, deep expertise in drug development, and wide-ranging experience in policy matters, demonstrated through her services as a senior executive in the biotechnology sector. She is a physician-executive with extensive industry knowledge garnered through her scientific and medical roles and experience as a global senior leader across multiple disease areas and all stages of drug development. She provides our board of directors with in-depth knowledge of our company gained during her various senior management roles within Vertex and through the day-to-day leadership of our executives as CEO. | |||
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 23
Other Public Company Boards:  None | Michel Lagarde | ||
Age: 50 Director Since: 2023 | Board Committees:  Member – Audit and Finance Committee | ||
Experience:  Executive Vice President and Chief Operating Officer at Thermo Fisher Scientific Inc. (“Thermo Fisher”), a supplier of analytical instruments, life sciences solutions, specialty diagnostics, laboratory, pharmaceutical and biotechnology services, since January 2022; Thermo Fisher is a supplier to Vertex  Executive Vice President at Thermo Fisher from 2019 through 2021  Senior Vice President and President, Pharma Services at Thermo Fisher from 2017 to 2019, joining Thermo Fisher as Senior Vice President, as a result of its acquisition of Patheon N.V., a pharma services company, in 2017  President and Chief Operating Officer of Patheon N.V. from 2016 to 2017  Managing Director of JLL Partners, a private equity firm focused on healthcare, from 2008 to 2016  Holds a bachelor’s degree in business administration from European University in Antwerp and an executive master’s degree in finance and control from the University of Maastricht and University of Amsterdam | |||
Key Skills and Qualifications: Mr. Lagarde has deep experience across numerous segments of the health care industry and markets around the world, as well as a successful track record of growing and scaling profitable businesses. He brings to our board of directors strong leadership experience in biotechnology and pharmaceutical development and commercial manufacturing services as well as financial expertise across several international markets. | |||
Other Public Company Boards:  None | Jeffrey Leiden | ||
Age: 68 Director Since: 2009 | Position:  Executive Chairman | ||
Experience:  Chief Executive Officer and President of Vertex Pharmaceuticals Incorporated from 2012 through March 2020  Chairman of Board of Directors of Vertex since 2012; previously served as lead independent director from 2010 through 2011  Managing Director at Clarus Ventures, a life sciences venture capital firm, from 2006 to 2012  President and Chief Operating Officer of Abbott Laboratories, Pharmaceuticals Products Group and a member of the board of directors of Abbott Laboratories from 2001 to 2006  Held several academic appointments from 1987 to 2000, including the Rawson Professor of Medicine and Pathology and Chief of Cardiology and Director of the Cardiovascular Research Institute at the University of Chicago, the Elkan R. Blout Professor of Biological Sciences at the Harvard School of Public Health, and Professor of Medicine at Harvard Medical School  Elected member of both the American Academy of Arts and Sciences and the Institute of Medicine of the National Academy of Sciences  Member of the board of directors of Quest Diagnostics Incorporated, a publicly traded medical diagnostics company, from 2014 to May 2019; member of the board of directors and non-executive Vice Chairman of Shire plc, a specialty biopharmaceutical company, from 2006 to 2012; Chairman of Revolution Healthcare Acquisition Corp., a special purpose acquisition corporation, from April 2021 to December 2022  Dr. Leiden received his M.D., Ph.D. and B.A. degrees from the University of Chicago | |||
Key Skills and Qualifications: Dr. Leiden possesses strong leadership qualities, demonstrated through his service as a senior executive in the biotechnology and pharmaceutical industries and as a life sciences venture capitalist, and has extensive knowledge of the science underlying drug discovery and development through his experiences as a distinguished physician, scientist, and teacher. As our former CEO and as a former senior executive at Abbott Laboratories, he brings a global perspective to our business and public policy issues facing our company. He also provides our board of directors with in-depth knowledge of our company and our corporate strategy. | |||
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 24
Other Public Company Boards:  MetLife Inc.  agilon health, inc. | Diana McKenzie | ||
Age: 59 Director Since: 2020 | Board Committees:  Member – Corporate Governance and Nominating Committee  Member – Science and Technology Committee  Member – Management Development and Compensation Committee | ||
Experience:  Chief Information Officer of Workday, Inc., a cloud-based financial and human capital management software company, from 2016 until April 2019  Held roles of increasing responsibility at Amgen Inc., a biotechnology company, for 12 years, most recently serving as Senior Vice President and Chief Information Officer  Held various leadership roles at Eli Lilly and Company, a pharmaceutical company, for 17 years, focused on drug development, reducing time to market and improving technology and security standards  Member of the board of directors of Change Healthcare, Inc., a publicly traded healthcare technology company, from August 2019 until it was acquired by United Health Group in October 2022  Holds a Bachelor of Science degree in Computer Information Systems from Purdue University and completed the Information Technology Management Program at University of California, Los Angeles and the CERT Certification for Cybersecurity Oversight from Carnegie Mellon’s Software Engineering Institute | |||
Key Skills and Qualifications: Ms. McKenzie has corporate leadership experience and industry knowledge that make her a valuable contributor to our board of directors. Her service as an executive and innovator in the biotechnology and technology industries and as a member of the board of directors of public companies involved in healthcare issues provide her with multiple perspectives on our industry. Ms. McKenzie brings extensive experience growing, scaling, and transforming global businesses in the healthcare and software industries. | |||
Other Public Company Boards:  None | Bruce Sachs | ||
Age: 64 Director Since: 1998 | Board Committees:  Chair – Management Development and Compensation Committee  Member – Corporate Governance and Nominating Committee | ||
Experience:  Partner Emeritus at Charles River Ventures (“CRV”), a venture capital firm; General Partner at CRV for more than 20 years, including more than 10 years as the Managing Partner  Executive Vice President and General Manager of Ascend Communications, Inc. from 1998 to 1999  President and Chief Executive Officer of Stratus Computer, Inc. from 1997 until it was acquired by Ascend Communications in 1998  Executive Vice President and General Manager of the Internet Telecom Business Group at Bay Networks, Inc. from 1995 to 1997  President and Chief Executive Officer of Xylogics, Inc. from 1993 until it was acquired by Bay Networks in 1995  Holds a B.S.E.E. in electrical engineering from Bucknell University, an M.E.E. in electrical engineering from Cornell University, and an M.B.A. from Northeastern University | |||
Key Skills and Qualifications: Mr. Sachs brings strong business judgment, honed through his experience developing business strategy as a senior executive and in venture capital, to our board of directors. Mr. Sachs has a deep understanding of our business and the global business environment along with expertise in the technology that supports our infrastructure and operations. In addition, Mr. Sachs has extensive business leadership experience, including service as a technology company CEO, as well as financial expertise. | |||
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 25
Other Public Company Boards:  None | Jennifer Schneider | ||
Age: 49 Director Nominee | |||
Experience:  Co-Founder and CEO of Homeward Health, Inc., a company committed to rearchitecting the delivery of health and care in rural America in partnership with communities, since 2022  President of Livongo Health, a biotechnology company, from December 2018 to October 2020, and served as the Chief Medical Officer from September 2015 to December 2018  Served in multiple leadership roles at Castlight Health, Inc., a healthcare navigation company, from 2010 to 2015, most recently as Chief Medical Officer  Member of the board of directors at Revolution Healthcare Acquisition Corp., a special purpose acquisition company, from March 2021 to December 2022  Member of the board of directors at Health Assurance Acquisition Corp., a special purpose acquisition company, from September 2020 until it liquidated in November 2022  Holds a B.S. in Biology from the College of the Holy Cross, an M.D. from Johns Hopkins School of Medicine, and an M.S. in Health Services Research from Stanford University | |||
Key Skills and Qualifications: Dr. Schneider has deep knowledge and expertise in healthcare and technology. She has led or held senior leadership roles in multiple companies focused on delivering innovative solutions in healthcare management. At Livongo, Dr. Schneider led the company through the largest consumer digital health initial public offering in history as well as its merger with Teladoc Health, the industry’s largest ever merger. Dr. Schneider brings extensive experience as a practicing physician and as a leader building healthcare companies that use technology to provide access to clinical services. | |||
Other Public Company Boards:  Denali Therapeutics  Schrödinger Inc. | Nancy Thornberry | ||
Age: 67 Director Since: 2023 | Board Committees:  Member – Science and Technology Committee | ||
Experience:  Founder and Chief Executive Officer of Kallyope, Inc. (“Kallyope”), a private biotechnology company, from November 2015 to October 2021, and served as Chair of Research and Development through December 2023  Self-employed as a consultant to companies in the biotechnology and pharmaceutical industries from August 2013 to October 2015  Served in roles on increasing responsibility at Merck & Co., Inc., a pharmaceutical company, for more than 30 years, most recently as Senior Vice President and Franchise Head, Diabetes and Endocrinology  Holds a B.S. in Chemistry and Biology from Muhlenberg College | |||
Key Skills and Qualifications: Ms. Thornberry has over 30 years of experience in the pharmaceutical and biotechnology industries and her scientific leadership has spanned across drug discovery, research & development, as well as business development. She brings to our board of directors leadership experience in critical scientific roles driving innovation at both public and private companies. Her industry experience, scientific acumen and strategic thinking brings great value to our board of directors. | |||
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 26
Other Public Company Boards:  None | Suketu Upadhyay | ||
Age: 55 Director Since: 2022 | Board Committees:  Chair – Audit and Finance Committee | ||
Experience:  Executive Vice President and Chief Financial Officer of Zimmer Biomet, a leading global innovator and manufacturer of orthopedic solutions, since July 2019  Senior Vice President, Global Financial Operations at Bristol-Myers Squibb from 2016 to June 2019  Executive Vice President and Chief Financial Officer of Endo International from 2013 to 2016  Previously served as interim Chief Financial Officer and Senior Vice President of Finance, Corporate Controller and Principal Accounting Officer of Becton Dickinson and Senior Vice President of Global Financial Planning and Analysis and Vice President and Chief Financial Officer of Becton Dickinson’s international business  Previously held a number of global finance and strategy roles across AstraZeneca and Johnson & Johnson, including Research and Development, Supply Chain, Commercial Operations and Business Development  Spent the early part of his career in public accounting with KPMG, earning his CPA designation and his CMA designation (each designation currently inactive)  Holds a Bachelor of Science in Finance from Albright College and an MBA from The Fuqua School of Business at Duke University | |||
Key Skills and Qualifications: Mr. Upadhyay has extensive experience in the health care industry in financial roles covering all major areas of a fully integrated life sciences business. His service as an executive in the pharmaceutical, hospital supply, and medical device industries provide him with multiple perspectives on our industry. His knowledge and expertise make him a valuable contributor to our board of directors and management. | |||
In each of the director nominee and continuing director biographies, that follow, we highlight the specific experience, qualifications, attributes, and skills that led the board of directors to conclude that the director nominee or continuing director should serve on our board at this time.
For all of the founder of Vertex and has been a director since our inception in 1989. He was our Chief Executive Officer from 1992 through May 2009. He was our Chairman ofabove reasons, our board of directors from 1997 until May 2006 and our President from our inception until December 2000, and from 2005 through February 2009. He was our Chief Scientific Officer from 1989 until May 1992. Prior to founding Vertex in 1989, Dr. Boger heldunanimously recommends that you vote FOR each of the position of Senior Director of Basic Chemistry at Merck Sharp & Dohme Research Laboratories in Rahway, New Jersey, where he headed both the Department of Medicinal Chemistry of Immunology & Inflammation and the Department of Biophysical Chemistry. Dr. Boger holds a B.A. in chemistry and philosophy from Wesleyan University and M.S. and Ph.D. degrees in chemistry from Harvard University.nominees.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 27
| Back of Contents | |
We are committed to good corporate governance and integrity in our business dealings. Our governance practices are documented in our Statement of Corporate Governance Principles, which addresses the role and composition of our board of directors and the functioning of the board and its committees. You can find our governance documents, including our Statement of Corporate Governance Principles, charters for each committee of the board, and our Code of Conduct, on our website www.vrtx.com under “Investors—Corporate Governance—Governance Documents.”
Our board of directors has determined that eightnine of our nine directorseleven director nominees qualify as “independent” under the definition of that term adopted by The Nasdaq Stock Market LLC or Nasdaq. These(“Nasdaq”). In addition, Mr. Kearney, one of our current directors who is not standing for re-election at our 2024 annual meeting of shareholders, was determined to be an independent director. Our independent director nominees are Dr. Bhatia, Mr. Carney, Dr. Boger,Garber, Mr. Kearney, Mr. Lee,Lagarde, Ms. McGlynn,McKenzie, Mr. Sachs, Dr. Schneider, Ms. UllianThornberry, and Mr. Young. Dr. Wayne Riley was an independent director prior to his resignation from our board in June 2015.Upadhyay. Our independent directors generally meet in executive session without management at each regularly scheduled board meeting.
Our board recognizes that one of its key responsibilities is to periodically evaluate the optimal leadership structure to ensure robust independent oversight of management and an engaged and effective board with complementary qualities, perspectives, and experiences. Given the dynamic and competitive environment in which we operate, the board believes that its optimal leadership structure may vary as circumstances warrant. The board values the flexibility to determine its optimal model for board leadership at any given time. As such, the board annually reviews its leadership structure and may choose a different leadership structure if circumstances should arise that lead it to believe that such structure would promote the long-term interests of our shareholders.
Our governing documents permit the roles of Chair of the board and CEO to be filled by the same or different individuals. To ensure robust independent leadership on the board, our corporate governance guidelines also require our independent directors.directors to elect a Lead Independent Director if the Chair of the board is a member of management or does not otherwise qualify as independent under the company’s director independence standards.
Currently, the board believes it is in the best interests of the company and our shareholders for the roles of Chair of the board and CEO to be separated, supported by a Lead Independent Director who has oversight responsibilities. Separating these positions at this time allows our CEO to focus on strategic decisions and our day-to-day business operations and performance, allows the Chair, our former CEO, to drive organization and effectiveness of the board in addition to his specifically delineated executive responsibilities, and allows our Lead Independent Director to lead the board in its fundamental role of providing advice to and independent oversight of management, including promoting communication between management and our board and supporting our board’s oversight of risk and other key governance matters. The board believes that this structure provides our board with independent leadership, while providing the benefit of having our former CEO chair regular board meetings and our current CEO participate in regular board meetings as a director.
The board annually evaluates whether combining or separating the roles of Chair of the board and CEO is in the best interests of the company and our shareholders. In its evaluation, the board considers a number of factors, including: (1) the individuals currently in the roles of CEO, Chair and Lead Independent Director and their record of leadership and performance in their roles; (2) current composition of the board; (3) the effectiveness of the policies, practices, and people in place to help ensure strong, independent board oversight; (4) the company’s performance and the effect the leadership structure could have on its performance; (5) the board’s performance and the effect the leadership structure could have on the board’s performance; (6) the Chair and Lead Independent Director’s performance in their respective roles; and (7) the views of our shareholders. A change in any of these factors could lead the board to determine in the future that it is more appropriate to have a different board leadership structure. Any such changes to our board leadership structure would be communicated to our shareholders through our annual proxy statement and as otherwise required by law.
Since 2020, Dr. Leiden has served as our Executive Chairman. In this role, Dr. Leiden not only serves as the Chair of our board, but also continues to have executive responsibilities with our corporate business development function, our cell and genetic therapy programs, and our external communications and government affairs activities, as described below. The board believes this leadership structure is currently in the best
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 28
interest of shareholders because Dr. Leiden, as a long-time executive of the company, has a vast knowledge and deep understanding of the company and its strategic plans, people, and operations. This company-specific experience, along with Dr. Leiden’s extensive knowledge of the industry and comparable organizations and broad involvement in drug discovery and development, as well as his distinguished tenure as Chair of the board since 2012 and wide-ranging experience in corporate governance and leadership, provide him with outstanding advising and oversight capabilities as Chair of the board. Dr. Leiden’s specific responsibilities include:
 | overseeing the corporate business development function; |
 | providing guidance on our cell and genetic therapy programs; |
 | supporting external communications, including with our shareholders; |
 | assisting the CEO with specific government affairs activities; |
 | collaborating with CGNC, the Lead Independent Director, and the CEO on the identification, evaluation, and recruitment of potential candidates for board membership and consideration of overall board composition; |
 | collaborating with CGNC and the Lead Independent Director on the recommendation to the board of individual directors to serve as members and chairs of each board committee; |
 | collaborating with CGNC and the Lead Independent Director on development and recommendation to the board of the annual self-evaluation process for the board and its committees; and |
 | collaborating with CGNC and the lead independent director on the oversight of the CEO succession planning process, including assistance in recruiting and identifying potential candidates for the CEO position. |
Our board believes that strong, independent board leadership is a critical aspect of effective corporate governance, and ourgovernance. Our corporate governance principlesguidelines require that if the chairChair is not an independent director, that the independent directors shall elect a lead independent director.Lead Independent Director. Since December 2011, Mr. Sachs and Ms. Ullian havehas served as our co-leadLead Independent Director or Co-Lead Independent Director. Mr. Sachs’ extensive business leadership experience as a CEO, a venture capitalist, and a director of the company, combined with his financial expertise, strongly positions him to provide independent directors. leadership of the board and its responsibility for identifying and assessing company risks and providing guidance on risk mitigation strategies.
The board recognizes the importance of appointing a strong Lead Independent Director to maintain a counterbalancing structure to ensure that the board functions in an appropriately independent manner and to hold management accountable for our continued success. As such, the responsibilities of the Lead Independent Director are designed to ensure that our independent directors are empowered to provide robust guidance to, and oversight of, management. Our Lead Independent Director’s responsibilities include:
 | calling and leading regular and special meetings of the independent directors; |
 | serving as a liaison between our management and independent directors; |
 | facilitating discussion and open dialogue among the independent directors, including with respect to the consideration of company risks and risk mitigation strategies; |
 | presiding at executive sessions of the independent directors; |
 | reviewing the planned dates for regularly scheduled board meetings and the primary agenda items for each meeting, which allows the Lead Independent Director to provide input and direction with respect to the matters that come before the board, including risk matters; |
 | providing board leadership if the Chair of the board or CEO may be (or may be perceived to be) in conflict with the best interests of the company and its shareholders; |
 | representing the board in certain communications with shareholders when requested and as appropriate; |
 | collaborating with CGNC, the Chair of the board, and the CEO on the identification, evaluation, and recruitment of potential candidates for board membership and consideration of overall board composition; |
 | collaborating with CGNC and the Chair of the board on the recommendation to the board of individual directors to serve as members and chairs of each board committee; |
 | collaborating with CGNC and the Chair of the board on development and recommendation to the board of an annual self-evaluation process for the board and its committees; and |
 | collaborating with CGNC and the Chair of the board on the oversight of the CEO succession planning process, including assistance in recruiting and identifying potential candidates for the CEO position. |
We believe thisour board structure provides our board independent leadership, while providing the benefit of having our chief executive officer, the individual with primary responsibility for managing our day-to-day operations, chair regular board meetings as we discuss key businessconsistent and strategic issues. Combined with the co-lead independent directors and experienced and independent committee chairs, this structure provides strong independent oversight of management.management, while also facilitating a collaborative and collegial environment for board deliberations and decision-making.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 29
| Back of Contents |
Our board of directors has established various committees, each of which has a written charter, to assist in discharging its duties: the audit and finance committee (“audit committee”), the corporate governance and nominating committee,CGNC, the MDCC, and the science and technology committee.committee (“S&T committee”). Each member of the audit committee, CGNC, MDCC and financeS&T committee corporate governance and nominating committee and MDCC is an independent director as that term is defined by the applicable SEC and Nasdaq.Nasdaq rules. The primary responsibilities of each of the committees are set forth below, and the committee memberships are provided in the table appearing on page 2032 of this proxy statement.
Each of the committees has the authority, as its members deem appropriate, to engage outside legal counsel or other experts or consultants in order to assist the committee in carrying out its responsibilities.
| Director (1) | Independence | Board | Audit and Finance | Corporate Governance and Nominating | Management Development and Compensation | Science and Technology | 2015 Attendance at Meetings (2) |
| Sangeeta N. Bhatia | X | ● | ● | ● | 88% | ||
| Joshua Boger | X | ● | Chair | 100% | |||
| Terrence C. Kearney | X | ● | Chair | ● | 100% | ||
| Yuchun Lee | X | ● | ● | ● | 100% | ||
| Jeffrey M. Leiden | Chair | 100% | |||||
| Margaret G. McGlynn | X | ● | ● | 100% | |||
| Bruce I. Sachs | X | Co-lead | ● | Chair | 100% | ||
| Elaine S. Ullian | X | Co-lead | Chair | ● | 95% | ||
| William D. Young | X | ● | ● | ● | 100% | ||
| 2015 Meetings | 7 | 8 | 5 | 7 | 5 | ||
The primary purposes of the audit and finance committee are to:
 | assist our board’s oversight of our accounting and financial reporting processes, including financial controls and audits of our financial statements; |
 | appoint, oversee, and replace, if necessary, our independent registered public accounting firm engaged for the purpose of preparing or issuing an audit report or performing other audit, review, or attestation services; |
 | review and discuss our annual audit, quarterly reviews and related disclosures, and oversee our internal audit function; |
 | review and make recommendations to our board concerning our financial structure, financial strategy, and risks; |
 | oversee our policies and programs and address risks related to our programs, our financial and accounting systems, accounting policies and investment strategies, internal audit function, and cybersecurity, and review material tax matters, including tax structure and strategies; |
 | address risks arising from related person transactions; and |
 | assist our board’s oversight of our Code of Conduct, related policies and procedures, and ongoing compliance matters as needed. |
Our independent registered public accounting firm reports directly to, and is held accountable by, our audit and finance committee in connection with the audit of our annual financial statements and related services.
Mr. Kearney,Upadhyay the chair of our audit and finance committee, is our “audit committee financial expert” as that term is defined in applicable rules and regulations of the SEC.SEC, and is independent according to the applicable listing standards of Nasdaq. In addition, each of the other members of the audit and finance committee are qualified to serve as an audit committee financial expert under the SEC's rules.
Our audit and finance committee reviews and if appropriate, recommends for approval or ratification by our board,approves all transactions with related persons that are required to be disclosed by us pursuant to Item 404(a) of Regulation S-K promulgated by the SEC, except for transactions, if any, related to the employment of executive officers, which would be
The corporate governance and nominating committee:
 | assist our board of directors in developing and implementing our corporate governance principles; |
 | recommend the size, composition, and leadership structure of our board and its committees; |
 | identify and recommend to our board qualified individuals for board membership, accounting for the appropriate balance of knowledge, experience, skills, expertise, tenure, and diversity; |
 | oversee the CEO succession planning process and assist the board in recruiting and evaluating potential candidates; |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 20
 | develop and recommend to our board an annual self-evaluation process to assess the effectiveness of our board and its committees, and coordinate this process; |
 | oversee risks related to the company’s governance structure; and |
 | review and make recommendations with respect to our committee charters. |
The primary purposes of the MDCC are to oversee the discharge of our board’s responsibilities relating to:
 | assess the overall compensation programs of the company and adopt a written statement of compensation philosophy and objectives; |
 | oversee and make recommendations to the board regarding compensation and development of our executives; |
 | recommend to the board (i) ratings for the company performance against company goals for the prior year and (ii) goals and weighting of goals for the next year; |
 | oversee risks associated with our compensation policies, management resources and structure, and management development and selection processes; |
 | oversee and make recommendations to the board regarding the compensation of our non-employee directors; |
 | review and approve our benefit and equity plans; and |
 | oversee and make recommendations to the board regarding the adoption, amendment, administration, and termination of any recoupment policy of the company. |
See
Compensation Discussion and Analysis—Detailed Discussion and Analysis below for a discussion of the MDCC’s role in overseeing executive compensation.The report of the MDCC appears on page 6673 of this proxy statement.
Our S&T committee assists our board of directors in its responsibilities relating to the oversight of our investment in pharmaceutical R&D. In furtherance of that oversight function, the S&T committee:
 | reviews and assesses our current and planned R&D programs and technology initiatives from a scientific perspective; |
 | oversees risks related to our R&D investments; |
 | assesses the depth and breadth of our scientific personnel and resources; and |
 | provides strategic advice to our board regarding emerging science and technology issues and trends. |
Messrs. Carney, Kearney, Mr.and Sachs, Ms. UllianMcKenzie and Mr. YoungYuchun Lee, a former member of our board of directors, served on the MDCC during all or a portion of 2015.2023. Each member of the MDCC was an independent director while serving on the MDCC. No memberNone of our boardthe members of directors who was a memberthe MDCC has been an officer or employee of the company. None of the members of our MDCC athad a relationship with the company or any timeof its subsidiaries during 2015 has ever been one of our employees or officers. No member of our board of directors who was a member of our MDCC at any time during 2015 has ever been a party to a transaction2023 that would be required to be disclosed pursuant to Item 404(a) of Regulation S-K prior to becoming a member of our MDCC.S-K. During 2015,2023, none of our executive officers served as a member of the board of directors or compensation committee of the board of directors, or performed the equivalent functions, of any entityanother company that has one or more executive officers serving as a member of our board or MDCC.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 31
During 2023, our board of directors met 5 times. Each of our incumbent directors attended at least 75% of the MDCC.
The following table provides information regarding our current board of directors. Jennifer Schneider has been nominated for our board of directors and therefore is not included in the table below. We expect that we will make additional changes later this year to our committee memberships resulting from the departure of Mr. Kearney following the 2024 annual meeting of shareholders.
| Director | Independence | Board | Audit Committee | CGNC | MDCC | S&T Committee | |||||||
| Sangeeta N. Bhatia |  |  |  |  | |||||||||
| Lloyd Carney |  |  |  |  | |||||||||
| Alan Garber |  |  |  |  | |||||||||
| Terrence C. Kearney |  |  |  |  | |||||||||
| Reshma Kewalramani |  | ||||||||||||
| Michel Lagarde |  |  |  | ||||||||||
| Jeffrey M. Leiden |  | ||||||||||||
| Diana McKenzie |  |  |  |  |  | ||||||||
| Bruce I. Sachs |  |  |  |  | |||||||||
| Nancy Thornberry |  |  |  | ||||||||||
| Suketu Upadhyay |  |  |  | ||||||||||
| 2023 Meetings | 5 | 7 | 5 | 5 | 4 |
 | = Member |
 | = Chair |
 | = Lead Independent Director |
Our board oversees an enterprise-wide approach to risk management, which is designed to support execution of our strategy and achievement of our goals to improve long-term operational and financial performance and generate long-term value for our shareholders. Our board believes that a fundamental part of risk management is understanding the risks that we face, adopting appropriate controls and mitigation activities for such risks, monitoring these risks, and responding to emerging developments with respect to such risks.
In performing its risk oversight responsibilities, the board relies, in part, upon the results and information gained through our annual Enterprise Risk Management (“ERM”) Program. The ERM Program is overseen by our Chief Risk Officer, who reports to our Chief Legal Officer, and is designed to identify key enterprise risks and ensure appropriate monitoring of, and controls over, those risks. As part of our ERM Program, we obtain input from our senior management and relevant subject-matter experts, evaluate industry trends and benchmarks, and consult external advisors as appropriate to identify the risks associated with our business based on likelihood of occurrence and potential impact to the business, as well as root causes of those risks. The ERM Program also assigns members of management responsibility for implementing controls and mitigations to reduce the likelihood or impact of each risk. The identified risks and related controls and mitigations are actively monitored by the board and regularly reviewed with senior management.
The board’s role in our risk management process also includes reviewing regular reports and updates from senior management on near-term, medium-term and long-term risks to the company. The board reviews and discusses strategic, operational, financial, compliance, legal, social (e.g., human capital management), environmental, governance, cybersecurity, and other risks. The board also receives updates from management regarding various enterprise risks related to our research, development, and commercialization plans, such as the competitive environment, manufacturing capabilities, product safety and quality, and drug pricing and reimbursement. These activities enable the board to understand and assess our risk environment, risk management, and risk mitigation strategies.
Near- and medium-term enterprise risks are afforded significant attention by the board. The board and its committees have the opportunity to provide input and direction as to the management of those risks in a variety of manners, including through the ERM Program review, regular operational and financial updates and review of development programs, regular updates from our Chief Legal Officer, internal audit updates, talent reviews and succession planning, updates on cyber-security, healthcare compliance updates, reviews of executive compensation, budget
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 32
reviews, and Technology Committee
Cross-functional members of management, including from our finance, accounting, investor relations, legal, compliance, information security, facilities, corporate communications, manufacturing, and disease strategy teams, work to ensure that material incidents and risks, including matters relating to significant actual or threatened litigation, significant regulatory matters and other matters that may have material financial, legal or reputational impacts on the company are timely reported to the board and senior management and disclosed to our shareholders, as appropriate. In addition, we disclose our significant environmental, social and governance progress, strategies, and commitments in our annual Corporate Responsibility Report.
While the board has the ultimate oversight responsibility for risk management, the board also manages risk through the division of responsibility within its committee structure, with each board committee being responsible for overseeing risk within its area of focus.
| Board Committee | Area of Risk Oversight | |
| Audit and Finance Committee |
Financial, accounting, disclosure, insurance, tax, investment, credit, disclosure controls and procedures, and other risks reviewed in its oversight of the internal audit function
Cybersecurity
Internal audit
Compliance program
Periodic reviews of relevant developments in disclosure requirements | |
| Management Development and Compensation Committee |
Executive compensation policies, practices, and goal setting, including whether such policies, practices, and goal setting balance risk-taking and rewards in an appropriate manner, align with shareholder interests, and are consistent with best practices
Company incentive compensation plans and equity-based plans
Stock ownership guidelines for executive officers and officers | |
| Corporate Governance and Nominating Committee |
Corporate governance
Board organization, membership, and structure
Board and CEO succession planning
Director independence | |
| Science and Technology Committee |
Assessments of planned R&D programs and technology initiatives
Emerging science and technology issues and trends |
At each regular meeting, or more frequently as needed, the board receives and considers committee reports, and such reports may provide additional detail on risk management issues and management’s response. The board and each committee also have the authority, in their sole discretion, to consult with and retain outside advisors and experts in connection with performance of its duties and responsibilities. Such outside advisors and experts are retained on a regular basis to assist the board and its committees in evaluating, managing, and anticipating risks, and include external auditors, legal counsel, compensation consultants, and business consultants, as appropriate. We have also established robust standards of business conduct that apply to all employees globally and provides numerous methods for employees to elevate risk concerns directly to management or through anonymous channels.
We have adopted a Code of Conduct that applies to all of our investment in pharmaceutical researchdirectors and development. In furtheranceemployees, including our CEO and chief financial and accounting officers. We routinely review our Code of Conduct and make updates, as necessary. Our Code of Conduct is available on our website www.vrtx.com under “Investors—Corporate Governance—Governance Documents.” Disclosure regarding any amendments to, or waivers from, provisions of the Code of Conduct that oversight function, the science and technology committee:
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 33
Vertex recognizes the importance of public policy in supporting our mission of creating transformative medicines for people with serious diseases. We engage with various policymakers and trade and industry groups to help promote an environment in which we can continue to innovate and develop transformative medicines for the benefit of patients with serious diseases.
Our board regarding emerging sciencehas oversight over our public policy activities and technology issues and trends; and
We meet all federal, state and local laws and reporting requirements governing corporate political contributions. We file quarterly reports listing the issues for which we conduct federal lobbying activities in compliance with the Honest Leadership and Open Government Act of Shareholders
We are a member of select industry and trade groups that are generally aligned with our business objectives and political contribution philosophy. These organizations represent the biotechnology industry and/or businesses more broadly in engaging with policy makers on issues that affect our industry. The industry and trade organizations to which Vertex paid more than $25,000 in dues have been disclosed on our website. Our governmental affairs executives regularly evaluate our participation in these organizations to ensure that they continue to be aligned with our contribution criteria and principles. We do not direct, nor do we have discretion over, how our membership dues are used and do not always agree with positions taken by these organizations and/or their members.
We do not make independent political expenditures or make payments to influence ballot measures.
At times, we may contribute to certain 501(c)(4) organizations that engage in lobbying or political activity. We will disclose such organizations annually on our website, and, to the extent available to us, the portion of those payments used for activities that are not deductible under Chapter 162(e) of the Internal Revenue Code. We do not contribute funds intended for elections to 501(c)(4) organizations.
We recognize that increasingly, investors are asking public companies to provide additional visibility regarding their political engagement and contributions and to provide information about accountability and oversight. We have made available on our website our political engagement principles, which provide transparency on our approach to political contributions, including lobbying activities. We have shared this with shareholders, engaged in productive dialogues on this topic, and update this information annually.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 21
| Back of Contents |
We have designed and implemented our compensation program for our non-employee directors to attract, motivate and retain highly experienced individuals who have strong industry knowledge, are committed to our values and goals, and who have the expertise and experience that we need to achieve those goals.
The compensation program for our non-employee directors and consistent with changes made to our executive compensation program, we modified the compensation program for our non-employee directors to reflect the fundamental changes in our business and financial profile. The changes include a transition from a share-based approach to a value-based approach in the granting of equity awards to our non-employee directors, reducing our reliance on stock options and increasing our cash fees. Under our revised program, the value of the equity awards to non-employee directors, as reported in the Director Compensation Table, will be reduced by more than 50% in 2016 as compared to 2015.
| Compensation Elements | |||||
| Cash | |||||
| Annual Cash Retainer | $ | 100,000 | |||
| Annual Committee Chair Retainer | Audit and Finance Committee | $ | 30,000 | ||
| Management Development and Compensation Committee | $ | 25,000 | |||
| Corporate Governance and Nominating Committee | $ | 25,000 | |||
| Science and Technology Committee | $ | 25,000 | |||
| Committee Membership Retainer | |||||
| Audit and Finance Committee | $ | 15,000 | |||
| Management Development and Compensation Committee | $ | 12,500 | |||
| Corporate Governance and Nominating Committee | $ | 10,000 | |||
| Science and Technology Committee | $ | 10,000 | |||
| Annual Lead Independent Director Retainer | $ | 40,000 | |||
| Equity | |||||
| Initial Equity Grant | A $400,000 value-based award in restricted stock units vesting after 12 months | ||||
| Annual Equity Retainer | On May 1 of each year, a $400,000 value-based award, which the directors can elect to receive in the form of:
 options that are fully-vested upon grant;
 restricted stock units that vest on the first anniversary of the date of grant; or
 a 50/50 mix of options and restricted stock units | ||||
Each of our non-employee directors is eligible to defer 50% or 100% of the cash and restricted stock unit portion of his/hertheir compensation set forth above and elect to receive deferred stock units that are paid out in common stock upon the earliest to occur of (i) termination of the non-employee director'sdirector’s service on our board of directors, (ii) a change of control, and (iii) the non-employee directorsdirector’s disability or death.
Our onlytwo employee director,directors, Dr. Leiden receives no separateand Dr. Kewalramani, do not receive compensation for histheir service as directors.
We annually review the compensation program for our non-employee directors. We did not make any material changes to the compensation program for our non-employee directors in such capacity.2023.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 35
| Director | Fees Earned or Paid in Cash | Stock Awards(1) | Option Awards(1) | All Other Compensation(3) | Total | |||||||||||||||
| Sangeeta N. Bhatia | $ | 135,000 | $ | 400,309 | $ | — | $ | 15,000 | $ | 550,309 | ||||||||||
| Lloyd Carney | $ | 134,484 | $ | 400,309 | $ | — | $ | 25,000 | $ | 559,793 | ||||||||||
| Alan Garber | $ | 125,000 | $ | 200,154 | $ | 200,076 | $ | 12,400 | $ | 537,630 | ||||||||||
| Terrence C. Kearney | $ | 133,146 | $ | — | $ | 400,037 | $ | — | $ | 533,183 | ||||||||||
| Michel Lagarde | $ | 23,913 | $ | 400,162 | $ | — | $ | 25,000 | $ | 449,075 | ||||||||||
| Yuchun Lee(4) | $ | 48,337 | $ | — | $ | — | $ | — | $ | 48,337 | ||||||||||
| Margaret G. McGlynn(4) | $ | 51,181 | $ | — | $ | — | $ | 25,000 | $ | 76,181 | ||||||||||
| Diana McKenzie(2) | $ | 127,796 | $ | 400,309 | $ | — | $ | 25,000 | $ | 553,105 | ||||||||||
| Bruce I. Sachs(2) | $ | 175,001 | $ | — | $ | 400,037 | $ | 25,000 | $ | 600,038 | ||||||||||
| Nancy Thornberry | $ | 7,337 | $ | 400,315 | $ | — | $ | — | $ | 407,652 | ||||||||||
| Suketu Upadhyay | $ | 124,354 | $ | 400,309 | $ | — | $ | 20,000 | $ | 544,663 | ||||||||||
| (1) | The amounts set forth under the captions “Stock Awards” and “Option Awards” in the table above represent the grant-date fair value for financial statement reporting purposes of the equity awards granted during 2023. The grant-date fair value of each option granted during 2023 was $114.92 utilizing the Black-Scholes option pricing model. |
| (2) | Ms. McKenzie and Mr. Sachs elected to defer their quarterly cash retainers, which were paid in deferred stock units, on each of the quarterly payment dates occurring on the 15th of the month following the quarter end in an amount equal to the dollar value of the cash amount that would have been paid on such date divided by the fair market value of a share of common stock on each such date. The per share fair market values of our common stock on each of those dates was $332.77, $350.79, $369.98 and $432.99. |
| (3) | Ms. Bhatia, Mr. Carney, Dr. Garber, Mr. Lagarde, Ms. McGlynn, Ms. McKenzie, Mr. Sachs and Mr. Upadhyay participated in the Vertex Foundation Matching Gift Program. |
| (4) | In 2023, Mr. Lee and Ms. McGlynn served on the board of directors until May 17, 2023. |
| Grant | Date | Shares | Exercise Price | Grant-Date Fair Value | ||||||||
| Annual Non-Employee Director - 100% Option Grants | May 1, 2023 | 3,481 | $ | 342.73 | $ | 400,037 | ||||||
| Annual Non-Employee Director - 50% Option Grants | May 1, 2023 | 1,741 | $ | 342.73 | $ | 200,076 | ||||||
| Annual Non-Employee Director - 100% Restricted Stock Unit Grants | May 1, 2023 | 1,168 | — | $ | 400,309 | |||||||
| Annual Non-Employee Director - 50% Restricted Stock Unit Grants | May 1, 2023 | 584 | — | $ | 200,154 | |||||||
| Initial Restricted Stock Unit Grant to Michel Lagarde | October 5, 2023 | 1,132 | — | $ | 400,162 | |||||||
| Initial Restricted Stock Unit Grant to Nancy Thornberry | December 5, 2023 | 1,136 | — | $ | 400,315 | |||||||
As of December 31, 2023, our non-employee directors had outstanding restricted stock units, deferred stock units and stock options to purchase our common stock as follows:
| Director | Outstanding Restricted Stock Units | Outstanding Deferred Stock Units | Outstanding Options (All Exercisable) | |||
| Sangeeta N. Bhatia | 1,168 | — | 1,938 | |||
| Lloyd Carney | 1,168 | — | — | |||
| Alan Garber | 584 | — | 26,413 | |||
| Terrence C. Kearney | — | — | 34,830 | |||
| Michel Lagarde | 1,132 | — | — | |||
| Diana McKenzie | 1,168 | 4,746 | — | |||
| Bruce I. Sachs | — | 14,643 | 40,793 | |||
| Nancy Thornberry | 1,136 | — | — | |||
| Suketu Upadhyay | 1,168 | 235 | — |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 36
We have stock ownership guidelines for our non-employee directors pursuant to which our non-employee directors should, within five years of becoming subject to the guidelines, achieve ownershiphold shares of shares of(a) our common stock, with(b) unvested restricted stock units, and/or (c) deferred stock units, having a value equal toof at least threefive times the annual cash retainer.
| Director | Fees Earned or Paid in Cash | Option Awards (1) | Total | |||||||||
| Sangeeta N. Bhatia | $ | 27,818 | $ | 1,691,118 | $ | 1,718,936 | ||||||
| Joshua Boger | $ | 62,500 | $ | 1,168,916 | $ | 1,231,416 | ||||||
| Terrence C. Kearney | $ | 80,000 | $ | 1,168,916 | $ | 1,248,916 | ||||||
| Yuchun Lee | $ | 60,000 | $ | 1,168,916 | $ | 1,228,916 | ||||||
| Margaret G. McGlynn | $ | 55,000 | $ | 1,168,916 | $ | 1,223,916 | ||||||
| Wayne J. Riley (until June 30, 2015) | $ | 35,000 | $ | 1,168,916 | $ | 1,203,916 | ||||||
| Bruce I. Sachs | $ | 100,000 | $ | 1,315,031 | $ | 1,415,031 | ||||||
| Elaine S. Ullian | $ | 100,000 | $ | 1,315,031 | $ | 1,415,031 | ||||||
| William D. Young | $ | 60,000 | $ | 1,168,916 | $ | 1,228,916 | ||||||
| Option Grant | Date | Shares | Exercise Price | Grant-Date Fair Value | |||||||
| Annual Non-Employee Director Grants | June 1, 2015 | 20,000 | $ | 127.54 | $ | 1,168,916 | |||||
| Annual Grants to Co-lead Independent Directors | June 1, 2015 | 2,500 | $ | 127.54 | $ | 146,115 | |||||
| Initial Grant - Sangeeta N. Bhatia | June 4, 2015 | 30,000 | $ | 126.68 | $ | 1,691,118 | |||||
| Director | Exercisable Options | Total Outstanding Options | ||
| Sangeeta N. Bhatia | 3,750 | 30,000 | ||
| Joshua Boger | 1,067,400 | 1,067,400 | ||
| Terrence C. Kearney | 60,375 | 60,375 | ||
| Yuchun Lee | 79,042 | 84,667 | ||
| Margaret G. McGlynn | 80,000 | 80,000 | ||
| Bruce I. Sachs | 120,000 | 120,000 | ||
| Elaine S. Ullian | 67,500 | 67,500 | ||
| William D. Young | 51,250 | 70,000 | ||
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 24
We are committed to operating our business responsibly and disclosing our progress to stakeholders on an annual basis. Our progress and efforts with respect to environmental, social, and governance topics, including community engagement and workplace practices, were recognized broadly in 2023. A selection of awards and recognitions include Fortune 100 Best Companies to Work For® 2023, Forbes’ Best Employers for Diversity, PEOPLE® Magazine’s 100 Companies That Care, Points of Light’s Civic 50, Science Magazine’s Top Employers, Seramount’s 100 Best Companies, and many others.
Our corporate responsibility priorities relate to four objectives fundamental to our business: improving the lives of people with serious diseases, fostering a culture of innovation, integrity, and inclusion; carefully managing our operations and environmental footprint; and making a positive impact in the communities where we are located.
 | Improve the Lives of People with Serious Diseases |
We are relentless in our pursuit to create transformative medicines for people with serious diseases. This has been our singular purpose for more than 30 years. We involve the voices of people living with the diseases early in our drug discovery and development processes. We listen, learn and incorporate the patient voice in our decisions and our R&D, every step of the way.
Our unique approach to building drug discovery programs has led to the development of four medicines that treat the underlying cause of CF, and the first-ever approved CRISPR-based gene edited cell therapy for SCD and TDT. At the end of 2023, our CF medicines were reimbursed or accessible to patients in more than 60 countries. In the U.S., more than 99 percent of eligible patients have access to TRIKAFTA through public and private insurance. We also have initiated a pilot program in lower-income countries to provide our latest triple combination therapy at no cost to eligible people with CF. In the geographies where CASGEVY is approved, we are actively working with key commercial and government payers and policymakers with the goal of securing rapid access for eligible patients with SCD and TDT.
We recognize the importance of going beyond R&D to engage in advocacy, awareness, and community support and are deeply committed to understanding the challenges and unmet needs of patients. We support programs and initiatives designed to raise disease awareness, educate health care professionals, strengthen R&D, and provide support to nonprofit organizations and patients. In 2023, we sponsored numerous programs and initiatives, including the Cystic Fibrosis Research Institute’s (“CFRI”) National CF Education Conference, the Rock CF Foundation’s Boltcast program, the Sickle Cell Community Consortium’s Warriors Convention and Caregiving Summit, the SCD Partnership, and the American Kidney Fund’s APOL1 Education Campaign.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 38
| Back of Contents |
 | Foster a Culture of Innovation, Integrity, and Inclusion |
Our success depends on nurturing a culture and team that values innovation, integrity and inclusion. Our culture of high ethical standards and integrity is one of the key components to our success, and each employee is responsible for upholding and demonstrating ethics and integrity in our work every day. Our Code of Conduct, anonymous compliance hotline, and robust investigation process are in place to help ensure that we are adhering to the highest standards of ethics and accountability.
We also are committed to building an outstanding, dedicated, and passionate team at Vertex, and fostering a culture that values inclusion, diversity, and equity. We believe that each employee brings unique perspectives and strengths, and by embracing these strengths, we can do our best work for patients. We focus on recruiting, retaining, and developing employees from a diverse range of backgrounds and view diversity through a broad lens that encompasses culture, backgrounds, experiences, and worldview to foster creativity and innovation.
Seven of our eleven director nominees are women and/or from underrepresented ethnic and racial groups. As of December 31, 2023, women represented 55% of our global workforce. In the U.S., approximately 41% of our workforce and 45% of new hires in 2023 were from underrepresented ethic and racial groups. As of December 31, 2023, approximately 40% of our global leadership (vice president and above) were women and 21% of U.S. leadership were from underrepresented ethnic and racial groups.
To promote our employees’ continued well-being and development, we offer a variety of inclusive benefits and opportunities. We also provide our employees with career development and advancement opportunities, including job rotations, mentoring, and managerial training. We are committed to identifying and developing our next generation of leaders and have developed programs focused on manager excellence, talent and succession for critical roles in our organization.
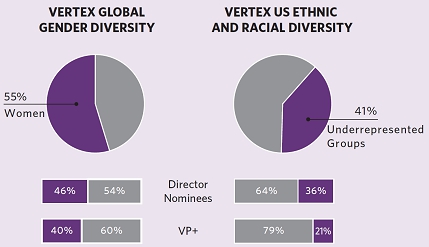
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 39
 | Carefully Managing Our Operations and Environmental Footprint |
We are committed to limiting our environmental impacts and to operating our business in a sustainable manner. We make strong local efforts to reduce our impact on the environment.
From 2022 to 2023, we reduced absolute GHG emissions by 3.5%. Building on this achievement, in 2023 we established a new target to reduce our Scope 1 and Scope 2 GHG emissions by 42% by 2032 from a 2022 baseline. 49% of our global energy comes from renewable energy sources and we continue to source 100% renewable electricity for our U.K.-based international headquarters and R&D facility. We also have ongoing efforts to minimize waste across all of our sites through employee engagement initiatives, vendor takeback programs and improved product distribution processes. Many of our facilities around the world are highly efficient, sustainable buildings. In 2023 our Jeffrey Leiden Center for Cell and Genetic Therapies was certified LEED (Leadership in Energy and Design) Platinum, the highest level of LEED certification for sustainable interior design and construction. Based on our efforts, we received a score of A- on the 2023 CDP Climate Change survey, demonstrating environmental leadership and a higher score than the global average of C.
We strive to offer our employees, contractors and visitors a healthy and safe work environment and continually seek ways to improve our practices. For example, each of our research and manufacturing sites has a Safety Committee, a forum where safety representatives, safety officers and Environmental, Health and Safety personnel meet regularly to discuss health and safety at the site and recommend preventive and corrective actions. We continually improve our performance by investing in safety programs and incorporating industry best practices to ensure that employees, contractors and visitors experience a healthy and safe work environment.
We also focus on the safety and security of our supply chain to protect our products and patients. Our medicines meet the highest safety, ethical and environmental standards and our teams work to prevent, detect and respond to instances of product diversion, tampering and counterfeiting, and to maintain the quality of our products for the patients who rely on them.
 | Making a Positive Impact in the Communities Where We are Located |
We are committed to making a positive difference in the communities where we are located. Our progress and efforts in corporate responsibility, community engagement, and workplace practices were recognized broadly in 2023. A selection of awards and recognitions include PEOPLE Magazine’s 100 Companies That Care, Points of Light’s Civic 50, and many others.
We support our communities through collaborations, donations, and volunteering across the world. In 2023, Vertex and the Vertex Foundation contributed more than $42 million in charitable giving. The Vertex Foundation, a 501(c)(3) nonprofit organization, seeks to improve the lives of people with serious diseases and contribute to our communities through education, innovation and health. In 2023, the Vertex Foundation provided grants in support of STEAM education, healthy families, and social innovation, with a focus on projects and organizations demonstrating a strong commitment to inclusion, diversity, and equity.
Our corporate giving extends and expands our long-term commitment to patients with serious diseases and our communities, with an on-going focus on STEAM education. For example, in 2023, we offered opportunities to nearly 3,000 students through our Learning Lab programs in Boston, San Diego, and Oxford, U.K., and, through the Vertex Foundation, provided 86 scholarships to people with CF and their family members who are pursuing higher education. The Vertex Foundation is also committed to supporting programs and initiatives designed to nurture the next generation of innovators, enable innovative solutions to community challenges, and support the quality of life of families with members living with serious diseases.
Volunteering and giving back are core to our culture. Throughout the year, we encourage our employees to participate in community service through the Vertex Volunteers program, including activities such as pro bono service conducted by members of our legal and compliance group and our 15th annual Day of Service. Last year, 60% of Vertex employees around the globe volunteered during Day of Service, contributing more than 8,200 volunteer hours to their local communities. Additionally, in 2023, we supported nearly 2,200 nonprofit organizations through the Vertex Foundation Matching Gift Program, which matches employee donations 1:1 to thousands of eligible nonprofit organizations.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 40
Our audit and finance committee is responsible for the appointment, compensation, and oversight of our independent registered public accounting firm. Ernst & Young LLP has been our independent registered public accounting firm since 2005, and we believe that the selection of Ernst & Young LLP as our independent registered accounting firm for the year ending December 31, 20162024 is in the best interest of our company and our shareholders.
In determining whether to reappoint our independent registered public accounting firm, our audit and finance committee undertakes an annual formal evaluation of the independent registered public accounting firm, during which it considers the quality of its discussions with, and the performance of, the lead audit partner, the audit team assigned to our account, the potential impact of changing our independent registered public accounting firm, the overall strength and reputation of the firm and issues pertaining to auditor independence, including fees that our independent registered public accounting firm receives for non-audit services.
Representatives of Ernst & Young LLP are expected to attend the annual meeting, will have the opportunity to make a statement if they desire to do so, and will be available to respond to appropriate questions from shareholders.
Although we are not required to have shareholders ratify the selection of Ernst & Young LLP.LLP, our board is submitting this proposal to our shareholders for ratification as a matter of good corporate practice. If our shareholders do not ratify the selection, our audit and finance committee will reconsider the selection of Ernst & Young LLP for the ensuing year, but may determine that continued retention of Ernst & Young LLP is in our company’s and our shareholders’ best interests. Even if the appointment is ratified, the audit and finance committee, in its discretion, may direct the appointment of a different independent registered public accounting firm at any time during the year if it determines
The audit and finance committee works with our management in order to negotiate appropriate fees with Ernst & Young LLP and is ultimately responsible for approving those fees. The following is a summary and description of fees for services provided by Ernst & Young LLP in
| Service | 2015 | 2014 | ||||
| Audit fees | $ | 1,750,000 | $ | 1,384,000 | ||
| Audit-related fees | 342,000 | 156,000 | ||||
| Tax fees | 1,645,000 | 1,030,000 | ||||
| All other fees | 1,995 | 1,995 | ||||
| Total | $ | 3,738,995 | $ | 2,571,995 | ||
| Service | 2023 | 2022 | |
| Audit fees | $ 4,650,000 | $ 4,652,200 | |
| Audit-related fees | — | — | |
| Tax fees | 1,160,000 | 2,936,000 | |
| All other fees | 7,000 | 4,000 | |
| TOTAL | $ 5,817,000 | $ 7,592,200 |
“
Audit“
Audit-relatedVERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 41
“Tax fees” fees”consisted of fees related to tax compliance, worldwide tax planning, and tax advice. The tax fees for 20152023 and 20142022 consisted of:
 | tax compliance and preparation fees, including the preparation of original and amended tax returns and refund claims, and tax payment planning of $896,000 and $1,806,000, respectively; and |
 | tax advice and planning fees of $264,000 and $1,130,000, respectively. |
“
All otherOur audit and finance committee has established a policy to pre-approve all audit and permissible non-audit services provided by our independent registered public accounting firm. Prior to the engagement of the firm for each year’s audit, management submits to our audit and finance
 | Audit fees include fees for audit work performed in the preparation of financial statements, as well as work that generally only our independent registered public accounting firm can reasonably be expected to provide, including comfort letters, statutory audits, consents, and attestation services. |
 | Audit-related fees relate to services for assurance and related services that traditionally are performed by the independent registered public accounting firm, including due diligence related to mergers and acquisitions, employee benefit plan audits, special procedures required to meet certain regulatory requirements, and consultation regarding financial accounting and/or reporting standards. |
 | Tax fees include fees for all services performed by the independent registered public accounting firm’s tax personnel except those services specifically related to the audit of our financial statements, and include fees in the areas of tax compliance, tax planning, and tax advice. |
 | All other fees are those associated with services not captured in the three preceding categories. |
Prior to the engagement of our independent registered public accounting firm, our audit and finance committee pre-approves these services by category of service. The fees are budgeted and our audit and finance committee requires the independent registered public accounting firm and management to report actual fees versus the budget periodically throughout the year by category of service. During the year, circumstances may arise when it may become necessary to engage the independent registered public accounting firm for additional services not contemplated in the original pre-approval. In those instances, our audit and finance committee requires that we obtain its specific pre-approval for these services.
The audit and finance committee may delegate pre-approval authority to one or more of its members. The member to whom such authority is delegated must report any pre-approval decisions to our audit and finance committee at its next scheduled meeting.
All of the services set forth above in the categories “audit-related fees,” “tax fees”fees,” and “all other fees” were pre-approved and none were approved by our audit and finance committee pursuant to Rule 2-01(c)(7)(i)(C), of Regulation S-X, which relates to the approval of a
The affirmative vote of a majority of the shares represented and entitled to vote on this matter is required for the approval of this proposal.
Our board of directors unanimously recommends that you vote FOR ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, 2024.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 42
| Back of Contents |
The Audit and Finance Committee of the Board of Directors (the “Audit Committee”) of Vertex Pharmaceuticals Incorporated (the “Company”), which consists entirely of directors who meetoversees the independencecompany’s accounting, auditing and experience requirements of the Securities and Exchange Commission and The Nasdaq Stock Market LLC, has furnished the following report:
The Audit Committee reviewed and discussed the company’s audited financial statements for the year ended December 31, 2023 with Ernst & Young is required to communicate to and discuss withthe company’s management, as well as Ernst & Young’s audit of the company’s internal control over financial reporting. In addition, the Audit Committee has discussed with Ernst & Young the matters that are required to be discussed by the applicable requirements of the Public Company Accounting Oversight Board (United States) Auditing Standard No. 16,
The Audit Committee discussed with Ernst & Young the firm’s independence, andalso has received from Ernst & Young the written disclosures and the letter concerning independence as required by Public Company Accounting Oversight Board Ethics and Independence Rule 3526 (Communicationthe applicable requirements of the PCAOB regarding Ernst & Young’s communications with Audit Committees Concerning Independence). This discussion and disclosure informed the Audit Committee of Ernst & Young’s relationships with the Companyregarding independence, and was designed to assist the Audit Committee in considering Ernst & Young’s independence. Finally, the Audit Committee reviewed andhas discussed with Ernst & Young the firm’s independence. The Audit Committee has also concluded that Ernst & Young’s provision of audit and non-audit services to the company is compatible with Ernst & Young’s independence from the Company’s management, the Company’s audited consolidated balance sheet as of
Based on the review and discussions noted above, the Audit Committee recommended to the company’s Board of Directors that the audited financial statements of operations, comprehensive income (loss), shareholders’ equity and noncontrolling interest, and cash flows for the year ended
Suketu Upadhyay (Chair)
Alan Garber
Terrence C. Kearney (Chair)
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 27
| Back of Contents |
Our compensation program is structureddesigned to attract, retain, and motivate talented and experienced leaders and reward our executive officers for the achievementindividuals across all areas of short- and long-term strategic and operational goals while avoiding inappropriate or excessive risk-taking. In 2015, our board of directors and the MDCC decided, based on fundamental changes in our business and financial profile and feedback we received from our shareholders, to adopt significant changes to our executive compensation program, and in particular, our equity compensation program that were implemented in early 2016.
In 2023, our annual advisory vote on executive compensation received support from approximately 89% of the votes cast at the annual meeting of shareholders. Based in part onWe believe this feedback,support is consistent with our shareholders’ understanding of our business model and the long-term value we made significant changesare creating. We plan to continue a high level of shareholder engagement regarding our equityexecutive compensation program, which we believe will allow usprogram.
Our focus is and continues to (i)be maintaining the strong link between our compensation programs and our ability to continue to attract, retain and motivatedevelop transformative medicines while delivering sustained company performance, with 90% of our named executive officers and (ii) address the concerns raised by our shareholders. As a result of these changes, our named executive officer's total equityNEO compensation as provided in the summary compensation tables is expectedlinked to decrease by 40% to 45% in 2016 under the new program as compared to our NEO's total equity compensation in 2015 under the prior program.
 | We maintained the base salary, target cash bonus, and target equity level for Dr. Kewalramani, based on a comparative analysis of companies in our peer group. |
 | Based on a comparative analysis of companies in our peer group, we maintained target compensation for Dr. Altshuler, Mr. Arbuckle and Mr. Wagner. |
 | Under Dr. Leiden’s amended employment agreement, he receives no cash compensation for his Executive Chairman role other than an annual cash payment intended to facilitate participation in the company’s benefit plans. Dr. Leiden will receive equity awards for his fifth year in his Executive Chairman role. |
 | Our outstanding performance in 2023 resulted in the board determining that we had achieved a leading rating for 2023 (150 out of a potential 150), with the payment of annual cash bonuses commensurate with this high level of performance. |
 | We maintained the mix of equity granted to our NEOs with 50% of the awards consisting of performance stock units that vest over time but only upon achievement of specific performance goals and 50% consisting of time-vesting restricted stock units. This mix rewards stock price appreciation and incentivizes long-term tenure. |
Our executive compensation program, including our performance and the compensation earned byof our named executive officers,NEOs, is discussed in greater detail in the
As required by Section 14(a) of the Exchange Act, our board of directors is asking that section, we discuss our executive compensation program and policies and explain the compensation decisions relating to our named executive officers for 2015. In addition, the compensation tables and related narratives, which begin on page 67 of this proxy statement, provide additional information regarding the compensation received by our named executive officers in 2015. In reviewing the compensation information included in this proxy statement, it is important to note that the equity compensation in these tables for 2015 reflects compensation received under the program we had in place prior to the changes implemented in early 2016.
RESOLVED, that the shareholders approve, on an advisory basis, the compensation of our named executive officers, as disclosed inpursuant to the rules of the Securities and Exchange Commission, including the Compensation Discussion and Analysis, section, the Compensation and Equity Tables and the related narrative executive compensation disclosures contained in this proxy statement.
The vote on this resolution is advisory in nature, and therefore will not bind us to take any particular action,binding on the board. However, our MDCC and board intend to consider carefully the outcome of the shareholder vote resulting from the proposal in makingwhen considering future decisions regarding our executive compensation program.
| Our board of directors unanimously recommends that you vote FOR the approval of the resolution set forth above. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 44
| Back of Contents |
We expect the following shareholder proposal will be presented for consideration at the 20162024 annual meeting of shareholders. The shareholder proposal will be voted on at the 2024 annual meeting of shareholders only if properly presented by or on behalf of the proponent. Our board of directors unanimously recommends a vote AGAINST thethis shareholder proposal for the reasons set forth following the proposal.
Vertex is not responsible for the content of this shareholder proposal or supporting statement.
John Chevedden, 2215 Nelson Avenue, No. 205, Redondo Beach, California 90278, an owner of the Stateat least $2,000 worth of New York, Division of Corporate Governance, 59 Maiden Lane - 30th Floor, New York, New York 10038, as the trustee of the New York State Common Retirement Fund, and as the administrative head of the New York State and Local Retirement System, who collectively are the owners 629,800 shares of our common stock as of December 17, 2015,5, 2023, has given notice that they or one or more of them, intend to present the following proposal for action at the 2016our 2024 annual meeting of shareholders.
Proposal 4 — Special Shareholder Meeting Improvement

Shareholders ask our board to take the steps necessary to amend the appropriate company governing documents to give the owners of a combined 10% of our outstanding common stock the power to call a special shareholder meeting.
To make up for our complete lack of a right to act by written consent we need the right of 10% of shares to call for a special shareholder meeting. Hundreds of major companies provide shareholders with the following resolution:
Certain companies, that shareholdersdo not provide for a shareholder right to act by written consent, have a more reasonable stock ownership threshold to call for a special shareholder meeting. Southwest Airlines is an example of a company that does not provide for shareholder written consent and yet provides for 10% of shares to call for a special shareholder meeting.
Since a special shareholder meeting can be useful in replacing a director, this proposal may be an incentive for the Vertex Pharmaceuticals urgedirectors to improve their performance and in turn improve the stock price.
Calling a special shareholder meeting is hardly ever used by shareholders but the main point of the right to call a special shareholder meeting is that it gives shareholders a Plan B option if management is not interested in good faith shareholder engagement. An incentive for management to engage with shareholders in good faith could be the alternative of a special shareholder meeting.
With the widespread use of online shareholder meetings it is much easier for management to conduct a special shareholder meeting and our bylaws thus need to be updated accordingly.
To anticipate a fake management objection to this proposal, shareholders who own 5% to 10% of a company are often the least likely shareholders to call for a special shareholder meeting.
Please vote yes:
Special Shareholder Meeting Improvement — Proposal 4
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 45
The board has carefully considered this proposal shouldand, for the reasons described below, has found it not prevent any director elected prior to the annual meeting held in 2016 from completing the term for which such director was elected.
As shown in the “Security Ownership of Certain Beneficial Owners and Management” table beginning on page 89, four of our shareholders separately own 5% or more of Vertex’s outstanding common stock. Lowering the threshold required to call a special shareholder meeting to 10% would provide two of such shareholders acting together to call a shareholder meeting. The board believes such a low threshold creates the risk that two shareholders may use this procedure to act unilaterally, or even threaten to do so to pressure the company, advancing their own special interests at the expense of other shareholders. The board further notes that adopting a 10% threshold would put the company in the minority of S&P 500 companies, as only approximately 12% of S&P companies have adopted a threshold of 10% or less.
Our board recognizes the importance of having strong corporate governance practices in place to ensure that the company is responsive to shareholder concerns. Our board further believes that shareholders should have a meaningful right to call special meetings in appropriate circumstances. As such, the company’s current by-laws allow shareholders who own 40% or more of the company’s outstanding common stock to call a special meeting. The by-laws allow shareholders to raise important matters with the company and demonstrates our commitment to effective corporate governance. Importantly, a 40% threshold provides a level of assurance that a reasonable number of shareholders consider a matter important enough to warrant a special meeting. Reducing the threshold to 10%, as proposed, could cause the company to spend significant resources on special meetings even if holders of up to 90% of the company’s shares do not believe that the issue warrants a special meeting.
If the shareholder proposal were adopted, a small minority of large shareholders—possibly even two shareholders as noted above— could force the company to devote outsized attention and resources to the special interests of that minority, at the expense of the company’s smaller shareholders. Maintaining our current threshold protects our smaller shareholders from the potentially narrow, short-term interests of a 10% shareholder, or a few shareholders totaling 10%. Lowering the threshold to 10% would allow such shareholders to call an unlimited number of special meetings, without regard to how direct costs and other burdens might affect the company’s future success or the interests of the vast majority of shareholders.
Preparing for, and holding, a special meeting is time-consuming and expensive. In connection with conducting a special meeting, the following reasons:
The board further believes that Vertex’s strong corporate governance practices make adoption of this proposal unnecessary. Our corporate governance practices provide the appropriate means to advance shareholder interests without the potential expense and risk of abuse that would come with lowering the threshold to call a special meeting. For example, our corporate governance and nominating committee.
 | Annual Election of Directors.At each annual meeting of shareholders, each director nominee is elected to hold office for a one-year term expiring at the next annual meeting of shareholders. |
 | Independent Board and Committee Leadership.Our board is led by a Lead Independent Director and each of our board Committees are chaired by, and composed solely of, independent directors. |
 | Shareholder Engagement.We have an ongoing, year-round shareholder engagement program and management regularly meets with shareholders who submit matters of concern or interest to our attention. |
 | Majority Vote Standard.Our by-laws provide for the election of directors by a majority votes cast in uncontested elections. |
 | Proxy Access.Our by-laws provide for proxy access which permits shareholders, or a group of 20 shareholders, owning 3% or more of our outstanding shares of common stock continuously for at least three years to nominate and include in our proxy materials nominees for director constituting up to 20% of the Board or two directors, whichever is greater, subject to the requirements set for in our by-laws. |
 | Majority Vote for Charter and By-Law Amendments.Our charter and by-law provisions do not have supermajority voting provisions - shareholders can approve binding charter and by-law amendments with a majority vote. |
| For all of the above reasons, our board of directors unanimously recommends that you vote AGAINST this shareholder proposal. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 46
| Back of Contents |
We expect the following shareholder proposal will be presented for consideration at the 20162024 annual meeting of shareholders. The shareholder proposal will be voted on at the 2024 annual meeting of shareholders only if properly presented by or on behalf of the proponent. Our board of directors unanimously recommends a vote AGAINST thethis shareholder proposal for the reasons set forth following the proposal.
Vertex is not responsible for the content of this shareholder proposal or supporting statement.
Arjuna Capital, 13 Elm Street, Manchester, Massachusetts 01944, representing its clients, John Silva and Shana Weiss through the Silva-Weiss Living Trust, which is the owner of 9,215at least $25,000 worth of shares of our common stock as of December 22, 2015,6, 2023, has given notice that it intendsthey intend to present for action at the 2016our 2024 annual meeting of shareholders the following resolution:
Whereas: Pay inequities persist across race and gender and pose substantial risks to companies and society. Black workers’ median annual earnings represent 77 percent of white wages. The shareholders askmedian income for women working full time is 84 percent that of men. Intersecting race, Black women earn 76 percent and Latina women 63 percent.1 At the boardcurrent rate, women will not reach pay equity until 2059, Black women in 2130, and Latina women in 2224.2
Citigroup estimates closing minority and gender wage gaps 20 years ago could have generated 12 trillion dollars in additional national income. PwC estimates closing the gender pay gap could boost Organization for Economic Cooperation and Development (OECD) countries’ economies by 2 trillion dollars annually.3
Actively managing pay equity is associated with improved representation. Diversity in leadership is linked to superior stock performance and return on equity.4 Minorities represent 40 percent of directorsVertex’s workforce and 22 percent of executives. Women represent 54 percent of the workforce and 33 percent of executives.5
Best practice pay equity reporting consists of two parts:
| 1. | unadjusted median pay gaps, assessing equal opportunity to high paying roles, |
| 2. | statistically adjusted gaps, assessing whether minorities and non-minorities, men and women, are paid the same for similar roles. |
Vertex Pharmaceuticals Incorporated to adopt a policy that in the event of a change in control (as defined under any applicable employment agreement, equity inventive plandoes not report quantitative unadjusted or other plan), there shall be no acceleration of vesting of any equity award granted to any senior executive officer, provided, however, that the board's Compensation Committee may provide in an applicable grant or purchase agreement that any unvested award will vest on a partial, pro rata basis up to the timeadjusted pay gaps. About 50 percent of the senior executive officer's termination, with such qualifications for100 largest U.S. employers currently report adjusted gaps, and an awardincreasing number of companies disclose unadjusted gaps to address the structural bias women and minorities face regarding job opportunity and pay.6
Racial and gender unadjusted median pay gaps are accepted as the valid way of measuring pay inequity by the United States Census Bureau, Department of Labor, OECD, and International Labor Organization. The United Kingdom and Ireland mandate disclosure of median gender pay gaps.7 For its United Kingdom employees, Vertex reports a median hourly pay gap of 20 percent and bonus gender pay gap of 26 percent.8
Resolved: Shareholders request Vertex Pharmaceuticals report on both quantitative median and adjusted pay gaps across race and gender, including associated policy, reputational, competitive, and operational risks, and risks related to recruiting and retaining diverse talent. The report should be prepared at reasonable cost, omitting proprietary information, litigation strategy and legal compliance information.
Racial/gender pay gaps are defined as the Committee may determine.
Supporting Statement: An annual report adequate for investors to assess performance could, with board discretion, integrate base, bonus and equity incentive plan as defined in item 402 of the SEC's Regulation S-K, which addresses elements of executive compensation to be disclosed to shareholders. This resolution shall be implemented so as not affect any contractual rights in existence on the datecalculate:
 | percentage median and adjusted gender pay gap, globally and/or by country, where appropriate |
 | percentage median and adjusted racial/minority/ethnicity pay gap, US and/or by country, where appropriate |
| 1 | https://www.census.gov/data/tables/time-series/demo/income-poverty/cps-pinc/pinc-05.html - par_textimage_24 |
| 2 | https://static1.squarespace.com/static/5bc65db67d0c9102cca54b74/t/622f4567fae4ea772ae60492/1647265128087/ Racial+Gender+Pay+Scorecard+2022+Arjuna+Capital.pdf |
| 3 | Ibid. |
| 4 | Ibid. |
| 5 | https://www.vrtx.com/sites/global/files/Vertex-IDE-Fact-Sheet.pdf |
| 6 | https://diversiq.com/which-sp-500-companies-disclose-gender-pay-equity-data/ |
| 7 | https://static1.squarespace.com/static/5bc65db67d0c9102cca54b74/t/622f4567fae4ea772ae60492/1647265128087/ Racial+Gender+Pay+Scorecard+2022+Arjuna+Capital.pdf |
| 8 | https://gender-pay-gap.service.gov.uk/EmployerReport/QPxr5sMh/2022 |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 47
The board has carefully considered this proposal is adopted, and, for the reasons described below, has found it shall apply only to equity awards made under equity incentive plans or plan amendments that shareholders approve after the date of the 2016 annual meeting.
We are highly focused on providing transparency and 2016 Proxy Statement | 32
Although the proposal is aimed at providing transparency with respect to pay equity, the unadjusted median pay ratio it requests is not a statistic that demonstrates whether women and employees belonging to underrepresented racial and ethnic groups are being paid fairly at Vertex for highthe roles they perform. This is because unadjusted median pay ratios merely compare the compensation midpoint for different groups of employees without adjusting for relevant factors that can explain variances in compensation. For instance, unadjusted median pay ratios do not account for factors such as job function, job level, education, experience, tenure, market pricing, labor force participation rates, performance, and geography, all of which legitimately impact compensation.
The board believes that an adjusted pay ratio, which takes into account such factors, provides a more accurate picture of pay equity and that our shareholders would not benefit from a broad, surrogate measurement of pay equity that would not provide any meaningful, additional information to help our shareholders evaluate the impact, effectiveness and equity of our pay practices.
At Vertex, our success is driven by the quality of our people, whom we believe are among the best and brightest in sustainable site development, water savings, energy efficiency, materials selectionthe life sciences industry. Our continued success depends on the collective strengths of our employees, and indoor environmental quality.
Vertex has adopted policies in support of our commitment to equal employment opportunity and fair treatment of employees. Our policies and practices with respect to pay equity include:
 | Policies designed to compensate employees in accordance with their job and level, without regard to gender, race, or other protected categories. |
 | Collection and review of external market and benchmark pay data along with internal pay data on an annual basis by job. Twice a year, during our mid- and year-end performance and compensation cycles, we analyze performance rating and pay decision distributions across groups to flag outliers and any other instances for further review. We conduct pay equity analyses using a variety of statistical methods to ensure that our pay practices are equitable at the group and individual levels. |
 | Computation of adjusted pay gaps across demographic groups, testing for differences in means and the shape of the distributions by demographic characteristics. In some small populations where there might be differences, we validate that they are explained by legitimate, nondiscriminatory, job-related factors such as function, experience, and tenure. At the individual employee level, we use multiple regression models to again validate that demographic variables do not influence compensation. |
 | Supplementing our internal analyses by engaging third-party experts (e.g., legal and compensation advisory firms), who conduct independent pay equity reviews that include advanced statistical analyses and for smaller populations where statistical analysis is not as useful, the third-party expert conducts cohort reviews to understand whether inequities exist. The analysis controls for a variety of legitimate, nondiscriminatory, job-related factors, including location, job family, job level, job performance, and relevant skills and experience. If we find an area of opportunity, we address it to ensure that employees are paid equitably for the same work. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 48
In our pursuit of scientific breakthroughs, we believe that good ideas come from people of all backgrounds. We are the most innovative, make the best decisions for patients and recruit the best talent when we have an inclusive, diverse and equitable workforce and culture. We value inclusion, diversity, and equity (“ID&E”) not only because it is the right thing to do, but also because it drives our business success.
We currently disclose gender and racial representation in our ID&E Fact Sheet, which also describes many of the programs we have to foster a culture that embraces ID&E. For example, our employee resource networks (“ERNs”) are voluntary, employee-led groups open to all employees that amplify the voices of our colleagues from traditionally underrepresented groups. These groups foster community and connection through education, leadership, development, networking, and volunteering, while also encouraging the kind of open dialogue that leads to stronger allyship across Vertex. Our employee resource networks first launched in 2016 and, since that time, membership has grown tremendously, from just over 100 members in a few locations to over 2,200 members across the globe in groups like IWILL, VIBE, PRIDE and BRAVE.
Our commitment to building and supporting ID&E in our workplace has been recognized by several leading organizations. These include a score of 100 on Human Rights Campaign’s Corporate Equality Index, and recognition on lists such as Fortune 100 Best Companies to Work For® 2023, Forbes Best Employers for Diversity, Seramount 100 Best Companies, STEM Workforce Diversity Top 50 Employers, People’s 100 Companies That Care, Newsweek America’s Most Responsible Companies, Science Magazine Top Employers, San Diego Business Journal Best Places to Work, and Boston Business Journal Best Places to Work.
As a result of our existing policies and procedures, as well as our new commitment to disclose adjusted pay gaps in 2025, the board believes that the adoption of this proposal — which includes an unadjusted pay gap component — is unnecessary and not in the best interests of our company or our shareholders.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 37
| Back of Contents |
Dear Fellow | ||||
| Shareholders, The Management Development and Compensation Committee’s stewardship of Vertex’s compensation programs is guided by Vertex’s mission of developing transformative medicines for people with serious | ||||
| 2023. We take seriously our role in the governance of compensation programs 2023 was a landmark year for Vertex as the company continued to successfully execute its business strategy. The research and development team made significant progress in advancing multiple programs, including those designed to treat cystic fibrosis (“CF”), sickle cell disease (“SCD”), transfusion-dependent beta thalassemia (“TDT”), acute and neuropathic pain, APOL1-mediated kidney disease, type 1 diabetes, myotonic dystrophy type 1, alpha-1 antitrypsin deficiency, and autosomal dominant polycystic kidney disease. More recently, in 2024, the company announced positive results from the Phase 3 clinical trials of its triple combination of vanzacaftor/tezacaftor/deutivacaftor (the “vanzacaftor triple”), which holds the potential to deliver even greater benefits to people with CF than TRIKAFTA, as well as from the Phase 3 clinical trials of VX-548 for the treatment of moderate-to-severe acute pain. With these advancements, Vertex’s research and development strategy is continuing to deliver a broad and deep pipeline of potentially transformative therapies, and thereby providing the opportunity for meaningful value for patients and shareholders. 2023 also marked an important pivot point for the company, as it commercially diversified into new disease areas with the historic regulatory approvals for CASGEVY, the first CRISPR gene-edited cell therapy to be approved in the world, as a potential one-time functional cure for people with SCD or TDT. Vertex also continued to expand its long-term leadership in CF by reaching younger age groups and additional geographies with existing medicines. With the recent data from the vanzacaftor triple and VX-548, Vertex is well-positioned to maintain its leadership in CF and further The company delivered outstanding financial performance in 2023, achieving CF net product revenues of $9.87 billion, an 11% increase compared to 2022, representing over a decade of consecutive years of double-digit growth. This revenue growth drove significant growth in earnings, and The company’s comprehensive success in 2023 reflects the execution of our differentiated corporate strategy championed by our senior management team. Consistent with these outstanding results, for 2023, our executives received above-target cash bonuses and payouts on performance stock unit awards based both on one-year business and financial goals. We believe | ||||
| performance. Looking ahead, we will continue to focus on | ||||
focus on creating long-term shareholder value. Sincerely, Bruce I. Sachs (Chair) Lloyd Carney Terrence C. Kearney Diana McKenzie | ||||
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 38
| Back of Contents | ||
This section discusses the principles underlying our policies and decisions with respect to the compensation of our "named executive officers"NEOs for 2023 and all materialthe factors we believe are relevant to an analysis of these policies and decisions. Our namedNEOs for 2023 are listed below.
| Name | Position |
| Reshma Kewalramani | Chief Executive Officer and President |
| Charles F. Wagner, Jr. | Executive Vice President, Chief Financial Officer |
| David M. Altshuler | Executive Vice President, Chief Scientific Officer |
| Stuart A. Arbuckle | Executive Vice President, Chief Operating Officer |
| Jeffrey M. Leiden | Executive Chairman |
Our CF medicines, TRIKAFTA/KAFTRIO, SYMDEKO/SYMKEVI, ORKAMBI, and KALYDECO, are transforming the lives of eligible people around the globe and continue to drive our financial performance.
 | Our CF net product revenues increased to $9.87 billion in 2023, an increase of 11% or more than $900 million, from our 2022 CF net product revenues. |
 | Our total R&D, AIPR&D and SG&A expenses increased to $4.8 billion compared to $3.6 billion in 2022. This increase was primarily due to higher AIPR&D, increased investment in support of multiple programs that have advanced to mid- and late-stage clinical development, and the costs to support new launches of Vertex’s therapies globally. |

VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 51
Our differentiated R&D strategy has delivered not only a decade-long leadership position in CF, but also the historic, global approvals for CASGEVY, the first CRISPR-based gene-edited cell therapy to be approved in the world, for people with SCD or TDT.
In CF, our goal is to bring highly effective treatments to all people with CF, and we began to do just that in January 2012 when KALYDECO was first approved to treat a CF population of approximately 1,000 people in the U.S. Since then, we have focused on growing the number of people eligible for our medicines, expanding access to our medicines in additional geographies, and seeking improved treatment options for all people with CF.
Today, our four approved CF medicines are collectively being used to treat nearly three quarters of the approximately 92,000 people with CF in North America, Europe, and Australia. Our CF medicines are used by people in over 60 countries and TRIKAFTA/KAFTRIO is now approved or accessible in more than 40 of these countries. In the near term, we expect our CF business to continue to grow as a result of (i) annualization of patients who recently initiated a CFTR modulator, (ii) label expansions, including into younger age groups and additional eligible mutations globally, (iii) expanded reimbursement, and (iv) the launch of the vanzacaftor triple, which will be a therapeutic option for those who have not yet initiated treatment with a CFTR modulator or who have discontinued from a CFTR modulator. In the mid- and longer-term, we foresee growth from (i) increases in the number of people living with CF, (ii) VX-522, a potential new therapy for the treatment of the more than 5,000 people with CF who cannot benefit from CFTR modulators, and (iii) next-generation CFTR modulator regimens.
In SCD and TDT, 2023 marked the commercial launch of CASGEVY, following approvals in the U.S., U.K., and Bahrain, and with approvals in the E.U. and Saudi Arabia following closely in early 2024. We estimate approximately 35,000 people with severe SCD or TDT could be eligible for CASGEVY in the U.S. and Europe, with additional eligible people in Saudi Arabia and Bahrain. Our global launch strategy for CASGEVY is focused on disease education and awareness for patients, caregivers, health care professionals, payors, and policymakers, as well as engagement with the scientific and medical community regarding CASGEVY clinical data. Our strategy is also focused on activating authorized treatment centers to ensure their readiness to treat patients and achieving access for patients through reimbursement agreements with governments and commercial payors, as well as through early access programs where applicable.
Since the beginning of 2023, notable progress includes:
 | The FDA, the European Commission, the MHRA, and Health Canada approved TRIKAFTA/KAFTRIO for the treatment of children with CF 2 to 5 years of age who have at least one F508del mutation in the CFTR gene. |
 | The FDA and the European Commission approved the use of ORKAMBI for children with CF from 12 months to less than 24 months of age who are homozygous for the F508del mutation. |
 | The FDA approved KALYDECO in children with CF from 1 month to less than 4 months of age. |
 | The approval of CASGEVY in the U.S., E.U., U.K., Saudi Arabia, and Bahrain for people 12 years of age and older with SCD or TDT. |
 | We have engaged with the Medicaid administrators in all 50 U.S. states, focused on the 25 states with the highest prevalence of SCD patients, and have confirmed pathways to reimbursement in nearly all 25 of these priority states. |
 | Approval by the French National Authority for Health of our request for the implementation of an early access program for the use of CASGEVY to treat eligible people with TDT from 12 to 35 years of age. |
 | We have begun engagement with payors in the U.K., E.U., Saudi Arabia and Bahrain, including engagement with the National Institute for Health and Care Excellence. |
 | Submission for approval of CASGEVY in both SCD and TDT in Switzerland. |
 | Activation of 16 authorized treatment centers in the U.S., four authorized treatment centers in Europe, and one in Saudi Arabia. |
 | Entry into an agreement with Synergie Medication Collective, a medication contracting organization, covering approximately 100 million people in the U.S., to provide access to CASGEVY. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 52
We are preparing for the following potential near-term launches of two new products:
 | Vanzacaftor triple in CF. We continued our strategy of serial innovation by completing three pivotal studies evaluating our once-daily triple combination CFTR modulator therapy vanzacaftor/tezacaftor/deutivacaftor. Results of the clinical trials demonstrate that this triple combination has the potential to provide additional clinical benefits beyond TRIKAFTA for people with CF who have at least one mutation in their CFTR gene responsive to CFTR modulators. This regimen also has the advantages of once-daily dosing and a lower royalty burden compared with TRIKAFTA. We expect to support the launch of the vanzacaftor triple with our existing commercial infrastructure. We expect to submit global regulatory filings for this triple combination by mid-2024, including in the U.S., the E.U., and Canada for people with CF 6 years and older. In the U.S., we will be using one of our priority review vouchers to shorten the regulatory review period from ten months to six months. |
 | VX-548 in acute pain. We completed three Phase 3 clinical trials for VX-548, a non-opioid, investigational selective NaV1.8 inhibitor, for the treatment of moderate-to-severe acute pain. Results of the clinical trials indicate that VX-548 could provide a transformative option for patients suffering from acute pain, based on the suboptimal benefit risk profile of existing agents, including the adverse effects and addictive potential of opioids, and the favorable benefit risk profile of VX-548. For our potential near-term commercial opportunity in acute pain, we are focused on the multi-billion dollar market arising from the estimated 80 million patients in the U.S. who are prescribed a medicine for their moderate-to-severe acute pain each year. More than two-thirds of patients receive acute pain prescriptions either during a hospital or ambulatory surgery center visit or at discharge; these prescriptions are concentrated in approximately 2,000 hospitals and 200 integrated delivery networks, which we believe we can reach with a specialty sales force. We plan to submit an NDA for the treatment of moderate-to-severe acute pain to the FDA by mid-2024. |
We invest in research and development to discover and develop transformative medicines for people with serious diseases, with a focus on specialty markets. Our research and development strategy combines advances in the understanding of human disease and the science of therapeutics to dramatically advance human health. This strategy was designed to deliver transformative medicines for serious diseases at high rates of speed and success, and it has delivered just that. Our success in moving novel product candidates into clinical trials, successfully completing pivotal development and obtaining marketing approvals offer multiple proof points of this strategy, and include TRIKAFTA/KAFTRIO, SYMDEKO/ SYMKEVI, ORKAMBI, and KALYDECO for CF, and CASGEVY for SCD and TDT. The strategy continues to be borne out by our pipeline, which includes potential future approvals of the vanzacaftor triple for the treatment of CF and VX-548 for the treatment of acute pain. Our approach to drug discovery has also yielded therapies that have demonstrated clinical proof-of-concept in additional disease areas, including neuropathic pain with VX-548, AMKD with inaxaplin, and T1D with VX-880, a stem cell-derived islet cell therapy.
Our research and development approach also includes pursuing multiple modalities tailored to the specific disease area target under investigation, using biomarkers that translate from the bench to the bedside, and advancing multiple candidates into clinical trials with the goal of bringing first-in-class, followed by best-in-class, therapies to patients. In addition to expanding our small molecule programs, we have also advanced an industry-leading portfolio of programs in cell and genetic therapies.
Our advancements across multiple disease areas and modalities include:
 | Cystic Fibrosis. We continue to pursue next-in-class, small molecule CFTR modulator therapies and have already identified next-in-class correctors and potentiators, as well as genetic therapies for people with CF who do not make full-length CFTR protein and, as a result, cannot benefit from our current CF medicines. For these more than 5,000 people with CF, in collaboration with Moderna, we are developing VX-522, a CF mRNA therapeutic designed to treat the underlying cause of CF in these people by enabling cells in the lungs to produce functional CFTR protein. We have completed dosing in the single ascending dose portion of the clinical trial for VX-522 in people with CF and initiated the multiple ascending dose portion of the trial. |
 | Sickle Cell Disease and Transfusion-Dependent Beta Thalassemia. We completed enrollment in two global Phase 3 clinical trials evaluating CASGEVY in people 5 to 11 years of age with SCD or TDT, and we are working on preclinical assets for gentler conditioning for CASGEVY, which could significantly broaden the eligible patient population. In addition, we are investigating small molecules for the potential treatment of SCD and TDT. |
 | Acute Pain. We completed a Phase 1 clinical trial of an oral formulation of VX-993, our next-generation NaV1.8 inhibitor, and plan to initiate a Phase 2 study for the treatment of moderate-to-severe acute pain in 2024. We also anticipate initiating a Phase 1 study of an intravenous formulation of VX-993 in 2024. |
 | Neuropathic Pain. We announced positive Phase 2 clinical trial results for VX-548 in DPN, a common form of chronic PNP, and will be initiating pivotal clinical development this year. We also initiated a Phase 2 clinical trial of VX-548 in lumbosacral radiculopathy, another type of PNP. In addition, we expect to initiate a Phase 2 clinical trial evaluating an oral formulation of VX-993 for the treatment of PNP in 2024. |
 | APOL-1 Mediated Kidney Disease. We completed enrollment in the Phase 2B dose-ranging portion of the clinical trial evaluating inaxaplin for the treatment of AMKD and have selected the dose for and initiated the Phase 3 portion of the Phase 2/3 pivotal clinical trial. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 53
 | Type 1 Diabetes. We completed Parts A and B, and completed enrollment in Part C of the Phase 1/2 clinical trial evaluating VX-880, an allogeneic, stem-cell derived, fully-differentiated, insulin-producing islet cell therapy, used in conjunction with standard immunosuppression, for the treatment of T1D in people with impaired awareness of hypoglycemia and recurrent hypoglycemic events. We have placed the study on a protocol-specified pause, pending review of the totality of the data by the independent data monitoring committee. The clinical trial for our second program in T1D, VX-264, in which the allogeneic stem-cell derived, fully-differentiated, insulin-producing islet cells are encapsulated and implanted in an immunoprotective device to obviate the need for immunosuppression, is a multi-part Phase 1/2 study. We have completed enrollment and dosing in Part A, and Part B of the clinical trial is underway in multiple centers and countries. |
 | Myotonic dystrophy type 1. We are exploring multiple approaches to address the underlying causal biology for DM1, including an oligonucleotide linked to a circular peptide, VX-670, which was in-licensed from Entrada. The IND for the Phase 1/2 clinical trial of VX-670 in people with DM1 has cleared, as have the regulatory submissions in Canada, the U.K. and multiple other geographies. The study has been initiated in Canada and is expected to initiate in other regions in the near term. |
 | Alpha-1 Antitrypsin Deficiency. We continue to enroll and dose Phase 1 clinical trials evaluating VX-634 and VX-668. |
 | Autosomal Dominant Polycystic Kidney Disease. We completed pre-clinical enabling studies for VX-407, our first-in-class small molecule corrector that targets the underlying cause of ADPKD in people with a subset of PKD1 genetic variants, in late 2023. The IND for VX-407 in the U.S. has cleared and we have initiated a Phase 1 clinical trial evaluating VX-407 in healthy volunteers in the U.S. |
 | In addition to the programs listed above, we have several earlier-stage research programs aimed at diseases that fit our R&D strategy, as well as follow-on programs in diseases already in the clinic. |
We will continue investing in our research and development programs and fostering scientific innovation by identifying additional product candidates through our internal research efforts and investing in business development transactions to access emerging technologies, products and product candidates.
The following chart represents our clinical stage programs and select pre-clinical programs.
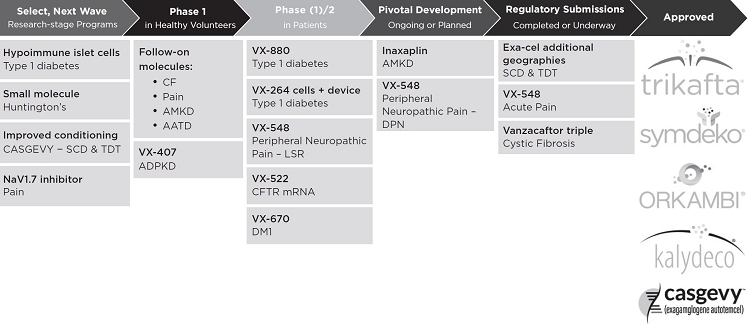
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 54
Driven by our financial performance and pipeline successes, our stock price increased 40.9% from $288.78 per share at the end of 2022 to $406.89 per share at the end of 2023. We believe biotechnology companies are best measured over the long term, as opposed to one-year or other shorter-term increments. The following charts show our total shareholder return relative to the NBI and S&P 500 index since the beginning of 2012, when our first CF medicine was approved, as well as our stock price performance over multiple periods. We believe the execution of our differentiated research and development approach and corporate strategy will continue to create shareholder value over the long term.
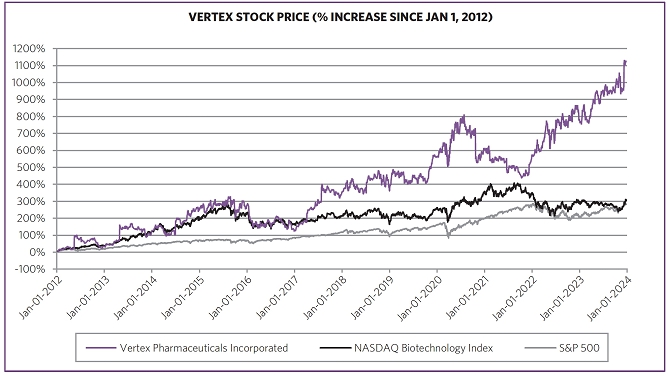
In 2023, our executive compensation program received substantial support from our shareholders, with approval by approximately 89% of the votes cast at the annual meeting of shareholders. We believe this support is consistent with our long-term shareholders’ understanding of our business model and the long-term value we are creating. Our executive compensation program is intended to align executive compensation with the company’s short- and long-term performance and to provide the compensation and incentives required to attract, motivate and retain our high-caliber executives who are crucial to Vertex’s long-term success. Our compensation program is highly performance-based, with 90% of our NEO compensation tied to performance. Retention of our talented executives is critical, as their outstanding performance has led to the company’s advancement and the creation of significant long-term shareholder value.
In 2023, our board of directors and MDCC reviewed our compensation programs and made the following key decisions:
 | Program Design: We maintained our compensation program design that directly ties pay with performance and that we believe has contributed to our short- and long-term successes. |
 | Base Salary: In 2023, based on a comparative analysis of companies in our Peer Group (described below), we maintained base salary compensation for Dr. Kewalramani, Dr. Altshuler, Mr. Wagner and Mr. Arbuckle. Under his employment agreement, Dr. Leiden does not receive a base salary for his role of Executive Chairman. |
 | Annual Cash Bonus: We maintained the target cash bonus, which is a percentage of base salary, for all of our NEOs. Dr. Leiden does not receive an annual cash bonus pursuant to his employment agreement. The company’s outstanding performance in 2023, as described above, resulted in the board determining that the company achieved a leading rating for 2023 (a rating of 150 out of a potential 150), and the payment of above-target annual cash bonuses. Please see Overview of Company Performance Rating & Achievement in 2023. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 55
 | Long-Term Equity Program: |
| – | In 2023, based in part on a comparative analysis of companies in our Peer Group, we maintained the target equity grant for Dr. Kewalramani and we maintained the target equity grants for our other NEOs. For his fourth year as Executive Chairman, Dr. Leiden received equity grants of $6.5 million pursuant to the terms of his employment agreement. Under his employment agreement, Dr. Leiden will continue to receive equity grants for the fifth year in his Executive Chairman role. | |
| – | We maintained our mix of equity granted under our compensation program with 50% of the awards consisting of PSUs that vest solely upon achievement of specific performance goals and 50% consisting of time-vesting RSUs. This mix rewards stock price appreciation and incentivizes long-term tenure at the company. The number of RSUs awarded may be adjusted to reflect an executive officer’s individual performance for the relevant performance period, and consequently are considered by the company to be performance-based awards. We replaced the performance-specific multiplier with a performance-specific multiplier range, as outlined under Annual Equity Awards on page 69 below. | |
| – | We achieved two of the three goals established for the 2021 non-financial based PSU awards and the maximum level of performance for the 2023 financial-based PSU awards, resulting in 100% and 200% payouts, respectively, under these awards in February 2024. | |
| – | In early 2024, the MDCC granted new awards of PSUs and established the financial and non-financial metrics that will determine whether and to what extent these awards become earned and vested. 50% of the PSUs, which vest annually in installments over three years measured from the date of grant, and are tied to net product revenue in 2024, while the remaining 50%, which cliff vest after three years, are tied to specific clinical and research milestones over a three-year period. |
We believe that a robust shareholder outreach program is an important component of maintaining our strong corporate governance practices. We strive for a collaborative approach with shareholders to solicit and understand a variety of perspectives and interests, and our practice has been to engage with our shareholders regularly over the course of the year.
During 2023, we solicited feedback regarding our corporate governance practices from our top 40 shareholders representing approximately 65% of our outstanding shares. Our integrated outreach team included leaders from our Investor Relations, Human Resources, Corporate Responsibility, Corporate Communications, and Legal teams, and we discussed numerous topics of shareholder interest, including our business strategy, R&D approach, diversity initiatives and metrics, employee engagement and development, corporate governance, political and lobbying disclosures, executive compensation, and environmental sustainability matters.
We continue to implement and maintain leading practices in our compensation program, shareholder outreach and related areas.
| What We Do | What We Don’t Do | |
| Caps on incentive awards | Excessive executive perquisites | |
| Multiple performance factors | Supplemental pension benefits for executives | |
| Range of awards; not all or nothing | Single-trigger vesting in connection with a change-in-control for equity awards | |
| Compensation recoupment (clawback) policy | Hedging or pledging or speculative transactions in our securities by directors and executive officers | |
| Balance of short- and long-term incentives (through annual cash bonuses and equity awards) | Re-pricing of stock options without shareholder approval | |
| Executive and Non-Employee Director Stock Ownership Guidelines | Allow payment of dividends on unvested performance shares or units | |
| Independent compensation consultant | 280G gross-ups (payments to offset excise taxes) | |
| Annual risk review | ||
| Pay for performance sensitivity and emphasis | ||
| Robust shareholder outreach |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 56
Our MDCC regularly reviews the elements of the individual compensation packages for our executive officers or NEOs, for 2015 were:
 | ||
 | align the interests of our executive officers with the interests of our shareholders as we seek to create value through the discovery, development, and commercialization of transformative | |
 | ||
Our executive officers have adopted significant changes tohad long and varied careers and possess diverse backgrounds and skills that make them extremely valuable members of our executive team and our company as a whole. The leadership, stability and commitment of this team have been instrumental in building Vertex into the company it is today: one with a leadership position in CF, a landmark gene-edited cell therapy for SCD and TDT, a broad, deep, and advancing pipeline, increasing revenues, and a strong financial profile. All of these factors position Vertex to achieve its strategic objectives in future years.
Our MDCC and board seek to connect the achievement of our strategic objectives with our compensation program in a number of ways, including through detailed and measurable company goals that underlie our annual cash bonuses and the performance goals that are used in particular,our equity awards. Our company goals address several key objectives, including increasing revenues from our current products, achievement of commercial manufacturing and R&D objectives, enhancing organizational capabilities, and maintaining financial strength. We believe this mix provides an appropriate balance between near- and long-term objectives and financial and organizational development objectives. These objectives are selected specifically because they are considered by our MDCC and board to be measurable milestones that our company must achieve if it is to maintain its significant revenue growth, superior profitability, and ability to continue creating value over the equity compensation component, which were implementedlong-term. Our MDCC and board expect to continue seeking balance in early 2016. The new equity compensation program reflects fundamental changesthe use of financial metrics and R&D goals to motivate our executive team to achieve financial objectives, while providing appropriate incentives for our management to continue to make investments in our business for the long term.
In determining compensation, we consider compensation paid by similar companies as a reference point among other factors, such as performance, competencies, experience and financial profilespecific role accountabilities, and feedback we received fromdo not solely benchmark at any particular level. Our MDCC retains flexibility to structure compensation based on good governance practices, our shareholders. While we continually engage in dialogue withobjectives of building our company and creating value for our shareholders, we increasedand, most importantly, discovering, developing, and delivering transformative medicines for patients who can benefit from them.
The MDCC has responsibility for overseeing the design, development, and implementation of the compensation program for our levelCEO, other executive officers, and senior leaders. The MDCC evaluates the performance of engagementour CEO and other executive officers. Our CEO, our Executive Chairman, and our human resources group assist the MDCC in responseevaluating the performance of our other executive officers, including the NEOs other than the CEO. Our CEO does not make any recommendations to the declineMDCC regarding CEO compensation and does not participate in support we received forthe portions of MDCC meetings or meetings of the board of directors when CEO compensation is discussed or determined. Similarly, our advisory say-on-pay proposal at our 2015 annual meeting. OverExecutive Chairman does not make any recommendations to the past year, we held specific discussionsMDCC regarding his compensation and does not participate in portions of MDCC meetings or meetings of the board of directors when Executive Chairman compensation is discussed or determined.
The members of the MDCC, each of whom is an independent director, make a recommendation regarding executive compensation with shareholders representing approximately 75%to the independent directors of the board, who together make final compensation decisions for the CEO and other executive officers based on these recommendations.
The MDCC (i) is directly responsible for the appointment and oversight of its compensation consultants, (ii) has the authority to determine the fees that we pay for services provided by such compensation consultants, and (iii) prior to engaging any compensation consultant, considers applicable factors potentially affecting the independence of the compensation consultant, including the factors set forth in Nasdaq Marketplace Rule 5605(d)(3).
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 57
Annually, the MDCC engages an independent compensation consultant to conduct an analysis of our outstanding stock.
The MDCC reviews Pearl Meyer’s independence in accordance with applicable Nasdaq and SEC rules. Based on this review, and other factors considered by the MDCC, the MDCC determined that Pearl Meyer’s work did not raise a conflict of interest.
In order to conduct a thorough assessment about elements of executive compensation on a competitive basis, the MDCC and our namedboard of directors considers information about the compensation practices of a representative group of companies with whom we compete for executive officers has increased duetalent (our “Peer Group”). We conduct a detailed analysis to our share-based approach,select companies for this Peer Group on the strong performancebasis of oursimilarity and complexity of business model. While revenue and increases in our share price. During this periodindustry are common peer selection criteria, we also matured from a development-stage company to a commercial-stage global biotechnology company with a strong financial profile and a clear path to sustained revenues and earnings growth. In early 2016, consistent with market practices for companies at our stage of development we transitioned from a share-based approach to a value-based approach for our long-term equity program.
| Factor Considered | What We Look For |
| Similar industry | Biotechnology or pharmaceutical industry |
| Importance of medicines to patients and society | Transformative medicines for serious diseases; therapeutics for unmet needs |
| Recognized focus on innovation | Breakthrough Therapy designations, priority review and/or other markers indicating unmet need |
| Global operations | Significant operations both within and outside the U.S. |
| Commercial operations | Marketing and selling approved medicines |
| Significant R&D investment | Greater than $2.2B or 25% of revenue |
| Number of employees | Greater than 1,200 employees |
| Market capitalization and significance to broader economy | Market cap at least ¼ our size and/or inclusion in S&P 500 or Nasdaq 100 |
| Labor market competitor | Companies we compete with for executive talent |
| Companies that use Vertex as a peer | Inclusion of Vertex in proxy reported peer group |
It is unlikely for companies to align on all of the factors listed above; therefore we look for companies meeting a majority of the valuecriteria although we place greater weight on companies focused on innovation and the importance of each award will have performance features (e.g., performance vesting or stock option awards); and
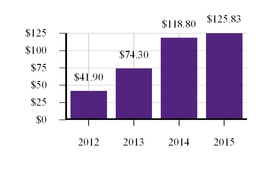
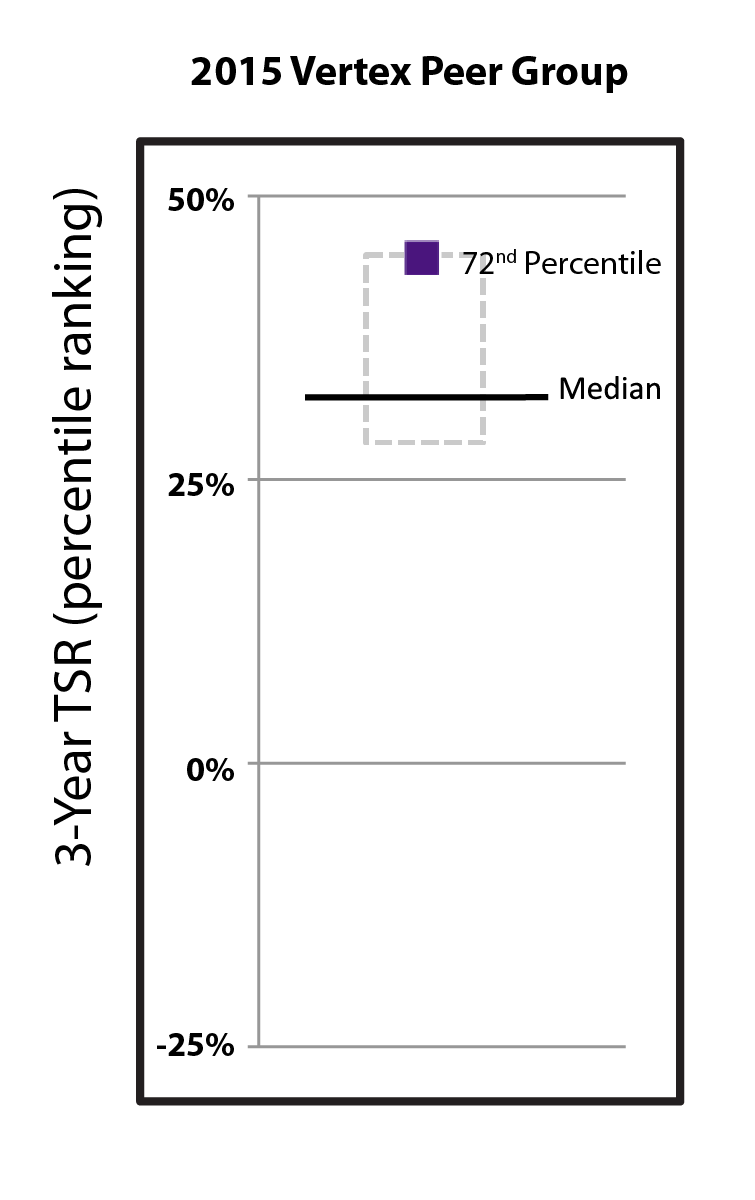
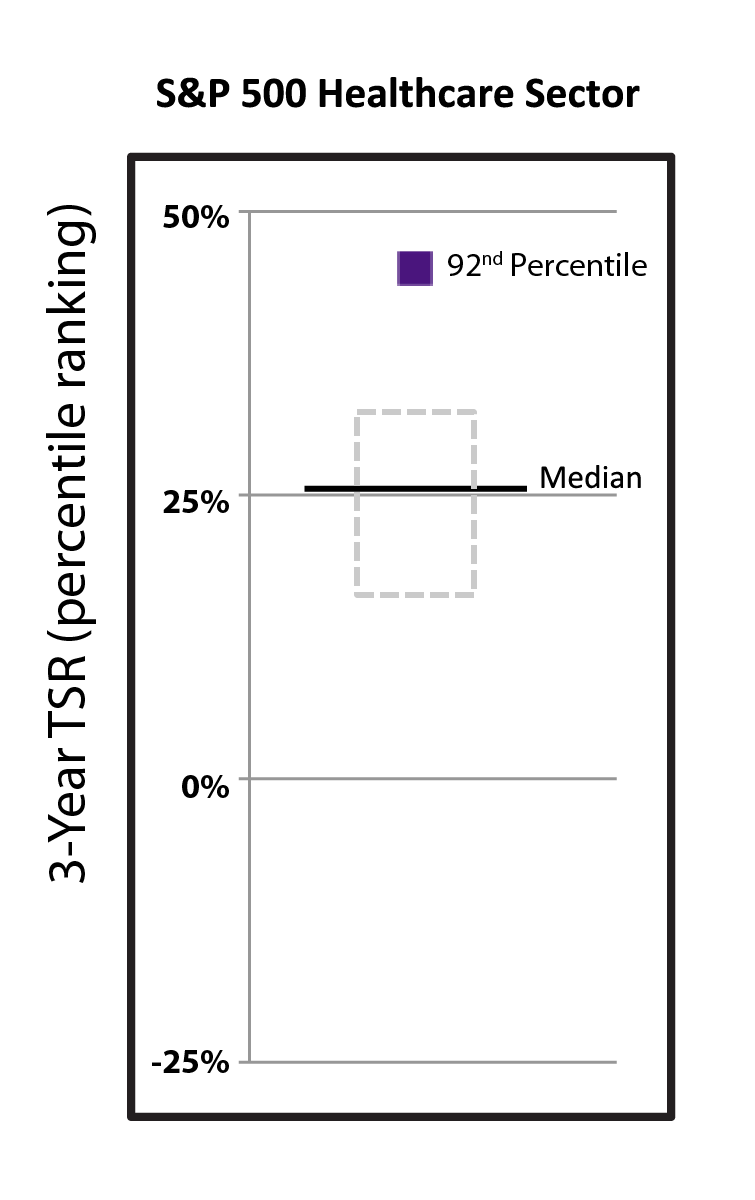
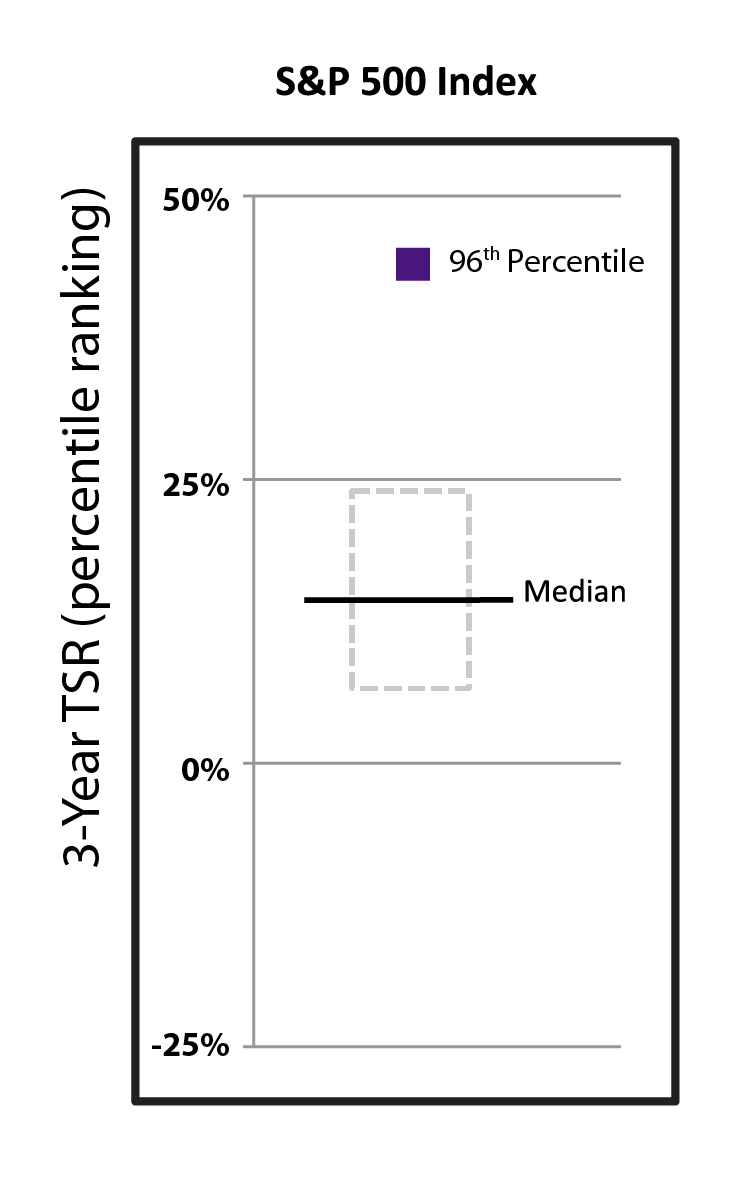
As a result of our exceptional performance in 2015, our board approved annual cash bonuses atthis analysis, and on the high-endbasis of the rangecriteria listed above, the MDCC approved the following comparator companies for each2023, which were the same comparator companies used in 2022. In 2024, we expect we will replace Seagen Inc. due to its acquisition by Pfizer.
| 2023 Peer Group | |
| AbbVie Inc. | Gilead Sciences, Inc. |
| Alnylam Pharmaceuticals | Incyte Corporation |
| Amgen, Inc. | Jazz Pharmaceuticals plc |
| Biogen, Inc. | Moderna, Inc. |
| BioMarin Pharmaceuticals, Inc. | Regeneron Pharmaceuticals, Inc. |
| Bristol-Myers Squibb Company | Seagen Inc. |
| Eli Lilly and Company | |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 58
We believe, based on our discussions with major shareholders, that the Peer Group identified by the MDCC is consistent with our shareholders’ views of our NEOs.
| Innovative and Importance of Medicines | |||||||||||||||||||||||
Company Information | R&D Expense(1) | Operational Focus | Orphan/ Unmet Clinical Need | Breakthrough Therapy Approvals and Regenerative Medicine Advanced Therapy Approvals(2) | Innovative Approved Drugs and Approved Regenerative Medicine Therapies in Last 13 Years(3) | Uses | Market Position | ||||||||||||||||
| Company | Industry | $ (millions) | % of Revenue | Global | Commercial | Nasdaq 100 | S&P 500 | ||||||||||||||||
| AbbVie | Biotech | $ | 8,453 | 16% |  |  |  | 7 | 9 |  | |||||||||||||
| Alnylam | Biotech | $ | 994 | 58% |  |  |  | 3 | 2 |  | |||||||||||||
| Amgen | Biotech | $ | 4,784 | 17% |  |  |  | 2 | 9 |  |  | ||||||||||||
| Biogen | Biotech | $ | 2,832 | 28% |  |  |  | 1 | 9 |  |  |  | |||||||||||
| BioMarin | Biotech | $ | 713 | 31% |  |  |  | 1 | 5 |  | |||||||||||||
| Bristol-Myers | Pharma | $ | 10,212 | 23% |  |  |  | 12 | 16 |  | |||||||||||||
| Eli Lilly | Pharma | $ | 13,113 | 38% |  |  |  | 4 | 12 |  | |||||||||||||
| Gilead | Biotech | $ | 5,718 | 21% |  |  |  | 6 | 9 |  |  |  | |||||||||||
| Incyte | Biotech | $ | 1,730 | 48% |  |  |  | 2 | 5 |  |  | ||||||||||||
| Jazz | Pharma | $ | 1,647 | 43% |  |  |  | — | 4 |  | |||||||||||||
| Moderna | Biotech | $ | 4,650 | 51% |  |  |  | — | 1 |  |  |  | |||||||||||
| Regeneron | Biotech | $ | 4,625 | 35% |  |  |  | 9 | 8 |  |  |  | |||||||||||
| Seagen(4) | Biotech | $ | 1,563 | 68% |  |  |  | 5 | 3 |  | |||||||||||||
| Vertex | Biotech | $ | 3,960 | 37% |  |  |  | 9 | 6 |  |  | ||||||||||||
| (1) | R&D Expense (including certain expenses related to intangible assets) and R&D Expense as a % of Revenue reflect the trailing data for the most recent four quarters as of December 31, 2023 per the S&P Capital IQ database. |
| (2) | Per the Center for Drug Evaluation and Research (CDER) Breakthrough Therapy Approvals report, which lists approvals for breakthrough therapy designated drugs and the Center for Biologics Evaluation and Research (CBER) Regenerative Medicine Advanced Therapy Approvals report, which lists approvals for breakthrough therapy designated regenerative medicines. |
| (3) | Innovative drugs and regenerative therapies in the last thirteen years include: VIEKIRA PAK, IMBRUVICA, VENCLEXTA, ORILISSA, MAVYRET, RINVOQ, SKYRIZI, QULIPTA and EPKINLY (AbbVie), ONPATTRO and GIVLAARI (Alnylam), AIMOVIG, BLINCYTO, XGEVA, PROLIA, KYPROLIS, PARSABIV, EVENITY, TEZSPIRE, and LUMAKRAS (Amgen), TECFIDERA, ALPROLIX, SPINRAZA, ELOCTATE, VUMERITY, ADUHELM, LEQEMBI, SKYCLARYS and QALSODY (Biogen), BRINEURA, PALYNZIQ, VIMIZIM, VOXZOGO and ROCTAVIAN (BioMarin), ABECMA, BREYANZI, SOTYKTU, CAMZYOS, ZESPOSIA, ONUREG, REBLOZYL, INREBIC, IDHIFA, EVOTAZ, OPDIVO, POMALYST, ELIQUIS, YERVOY, OPDUALAG and AUGTYRO (Bristol-Myers), CYRAMZA, EMGALITY, JARDIANCE, OLUMIANT, PORTRAZZA, RETEVMO, REYVOW, TALTZ, VERZENIO, MOUNJARO, JAYPIRCA and OMVOH (Eli Lilly), YESCARTA, SOVALDI, HARVONI, VEMLIDY, CAYSTON, ZYDELIG, BIKTARVY, VEKLURY and SUNLENCA (Gilead), JAKAFI, OLUMIANT, PEMAZYRE, RUXOLITINIB and ZYNYZ (Incyte), VYXEOS, DEFITELIO, ZEPZELCA and RYLAZE (Jazz), SPIKEVAX (Moderna), DUPIXENT, LIBTAYO, PRALUENT, EYLEA, ZALTRAP, INMAZEB, EVKEEZA and VEOPOZ (Regeneron), and PADCEV, TUKYSA and TIVDAK (Seagen). |
| (4) | Seagen Inc. was acquired by Pfizer in December 2023; financials reflect trailing twelve months as of September 30, 2023. |
We do not solely benchmark to a particular level of compensation relative to compensation levels at the Peer Group companies, but rather assess compensation levels after considerable deliberation about where each executive should fall in comparison with executives with similar responsibilities at the Peer Group companies. The MDCC looks at Peer Group information to confirm that our compensation levels are competitive with those of the Peer Group companies and consistent with our compensation philosophy. In addition, the MDCC reviews broader industry specific executive compensation surveys published by Radford, Mercer SIRS, and Willis Towers Watson, but does not make any material compensation decisions based on any particular company participants in such surveys.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 59
Due to the low representation of the Chief Operating Officer role within the Peer Group, Pearl Meyer supplemented the Peer Group with broader life science industry companies for purposes of analyzing competitive compensation for this role. Pearl Meyer reviewed companies within the broader life sciences industry and included all companies with a chief operating officer or similar role and greater than $2 billion revenues and greater than $10 billion market capitalization. The MDCC reviewed the compensation practices for the following broader life science industry companies when considering compensation for Mr. Arbuckle:
| Peer Group Companies | Life Science Companies |
| Amgen, Inc. | Bio-Rad Laboratories, Inc |
| Bristol-Myers Squibb Company | DexCom, Inc. |
| Jazz Pharmaceuticals plc | ResMed, Inc. |
| Thermo Fisher Scientific Inc. | |
| Zimmer Biomet Holdings Inc. |
Our practice is to target total direct compensation including base salary, target annual cash incentives, and target long-term incentive awards at market competitive levels depending upon the NEO’s responsibilities, expertise, and experience. At superior levels of performance, we aim for the design of our executive compensation program to result in actual total direct compensation at or above the seventy-fifth percentile of peer executive compensation. Each year we review the mix of elements of our executive compensation program to ensure they are appropriately designed in light of our goals to align the program with our business strategy, the competitive environment and our shareholders’ interests.
Our executive compensation program emphasizes a mix of long-term equity compensation awards to incentivize and reward those individuals who make the greatest contribution to company performance over time. For the NEOs, this means compensation is primarily in the form of equity and directly tied to changes in shareholder value over time. For our 2023 equity grants, we maintained our mix of equity awards, including PSUs and time-based RSUs.
As shown in 2015 included the sign-on equity grant he received when he became an employeefollowing charts, our compensation program places significant weight on performance-based compensation, with 90% of our NEO compensation tied to performance, or “at-risk” if performance is not achieved.
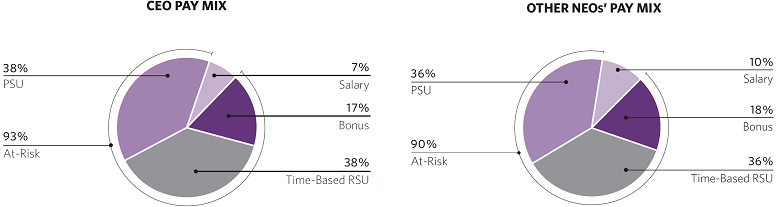
The charts above generally represent the values in January 2015.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 42
Performance-Based Value-Based Program
We have a performance-based program that is consistent with programs implemented by our peers and allows us to attract, retain, and motivate talented and highly experienced individuals across all areas of our business. We focus on the following performance-based elements:
| Compensation Element | Performance Link | |
| Annual Cash Bonus |  | Annual bonus dependent on both company and individual performance factors |
 | Grant date value of equity awards based on target award values by level with differentiation for individual performance | |
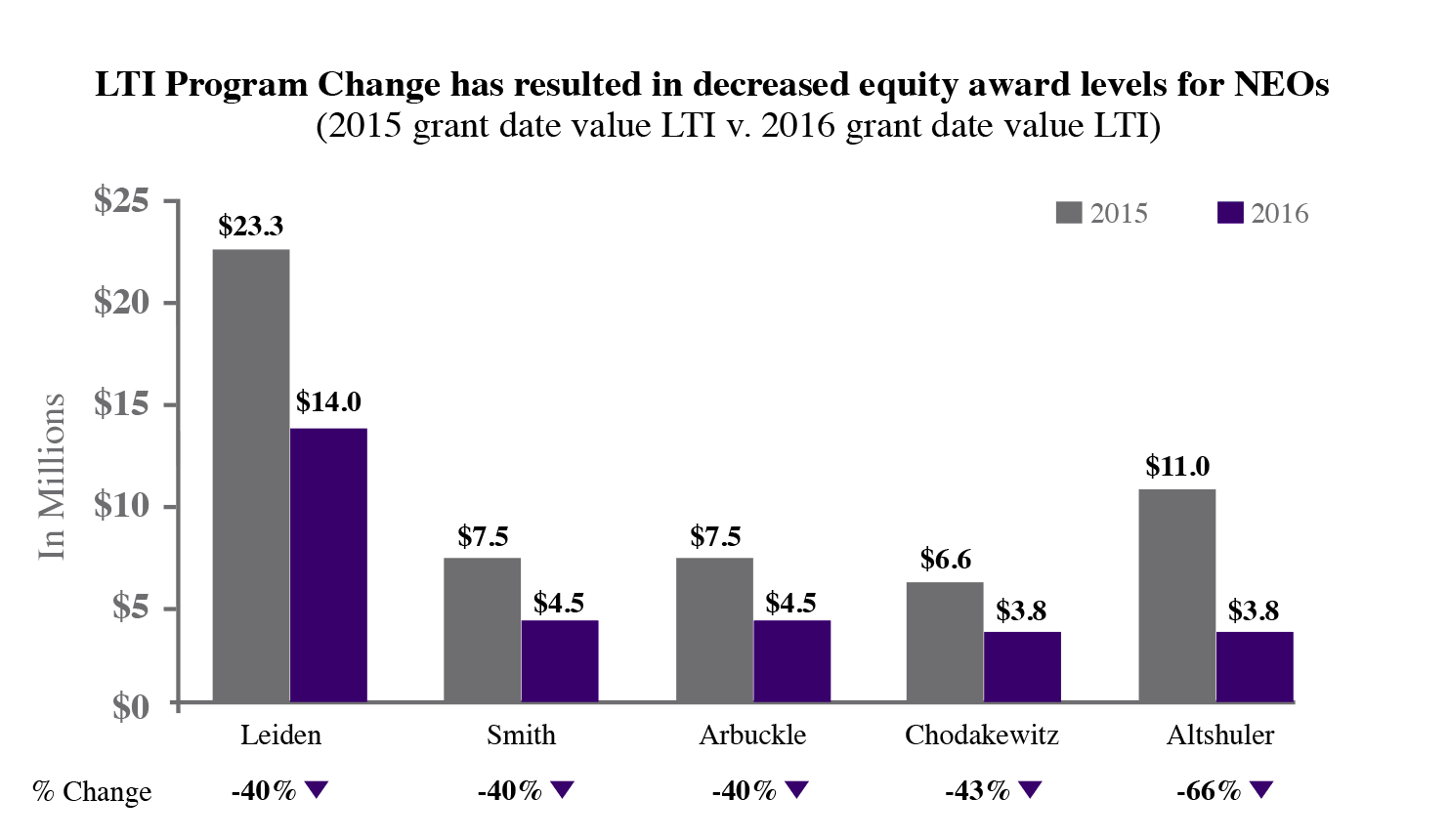





More specifically:
 | PSU Awards. Our CEO and executive vice presidents (“EVPs”) receive 50% of their annual equity compensation in the form of PSUs. 50% of the number of PSUs are eligible to vest based on the achievement of financial goals and the remaining 50% of the number of PSUs are eligible to vest based on the achievement of non-financial goals. The financial PSUs, if earned, vest in annual installments over a three-year period measured from the date of grant, and the non-financial PSUs, if earned, cliff vest after three years. The potential shares earned pursuant to these PSU awards range from 0% to 200% of the target number of shares, with the number of shares actually issued determined by the achievement of the financial and non-financial performance goals. The MDCC selected revenue and clinical development milestones as the performance goals applicable to the PSUs because the MDCC determined that these milestones are important, measurable metrics, the achievement of which would indicate successful execution toward our short- and long-term strategic objectives and build considerable shareholder value. |
 | Time-based RSUs. Our CEO and EVPs receive 50% of their annual equity compensation in the form of time-based RSUs that vest over a three-year period measured from the date of grant. With 50% of the annual long-term incentive award at risk pending successful execution of our strategic objectives through the grant of PSUs, we believe that it is important to have the remaining half of the annual long-term equity award focused on retaining our key executive talent. As a result, we believe time-based RSUs encourage retention and focus on long-term value creation thereby aligning with the interests of our shareholders. |
The MDCC recommends base salaries for our executive officers based on multiple factors, including a competitive market analysis on a position-by-position basis. Annually, the MDCC reviews a comparison of each executive’s prior year base salary and cash bonus opportunity, measured at the target level, to salaries and cash bonuses reported for executives with similar responsibilities at Peer Group companies. The MDCC does not solely benchmark to a particular level of compensation relative to compensation levels at the Peer Group companies. Instead, the MDCC’s judgment about where each executive should fall in comparison with executives with similar responsibilities at the Peer Group companies takes into account the executive’s general level of experience and capability, the significance of the executive’s job responsibilities to the achievement of our business strategy and company goals, and general performance over time, including demonstration of corporate values. On the basis of that information, including compensation at Peer Group companies, and taking into consideration the executive’s base salary for the previous year or years, the MDCC recommends an appropriate base salary for each executive officer, subject to final approval by our independent directors. Our current base salaries reflect each individual executive’s past and expected future contributions, performance, experience, specific responsibilities relative to peer benchmarks, and competitive positioning within the range around the median base salaries for our Peer Group companies' paycompanies.
Dr. Kewalramani’s base salary for 2023, as our CEO and performance. Our three-year TSR approximatedPresident, was maintained at $1.50 million, based on multiple factors, including her contributions as CEO and President, expected future contributions, experience and knowledge. We also maintained the 72
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 43
| Name | 2023 Base Salary | ||
| Reshma Kewalramani | $ | 1,500,000 | |
| Charles F. Wagner, Jr. | $ | 825,000 | |
| David M. Altshuler | $ | 825,000 | |
| Stuart A. Arbuckle | $ | 900,000 | |
| Jeffrey M. Leiden(1) | $ | — | |
| (1) | ||
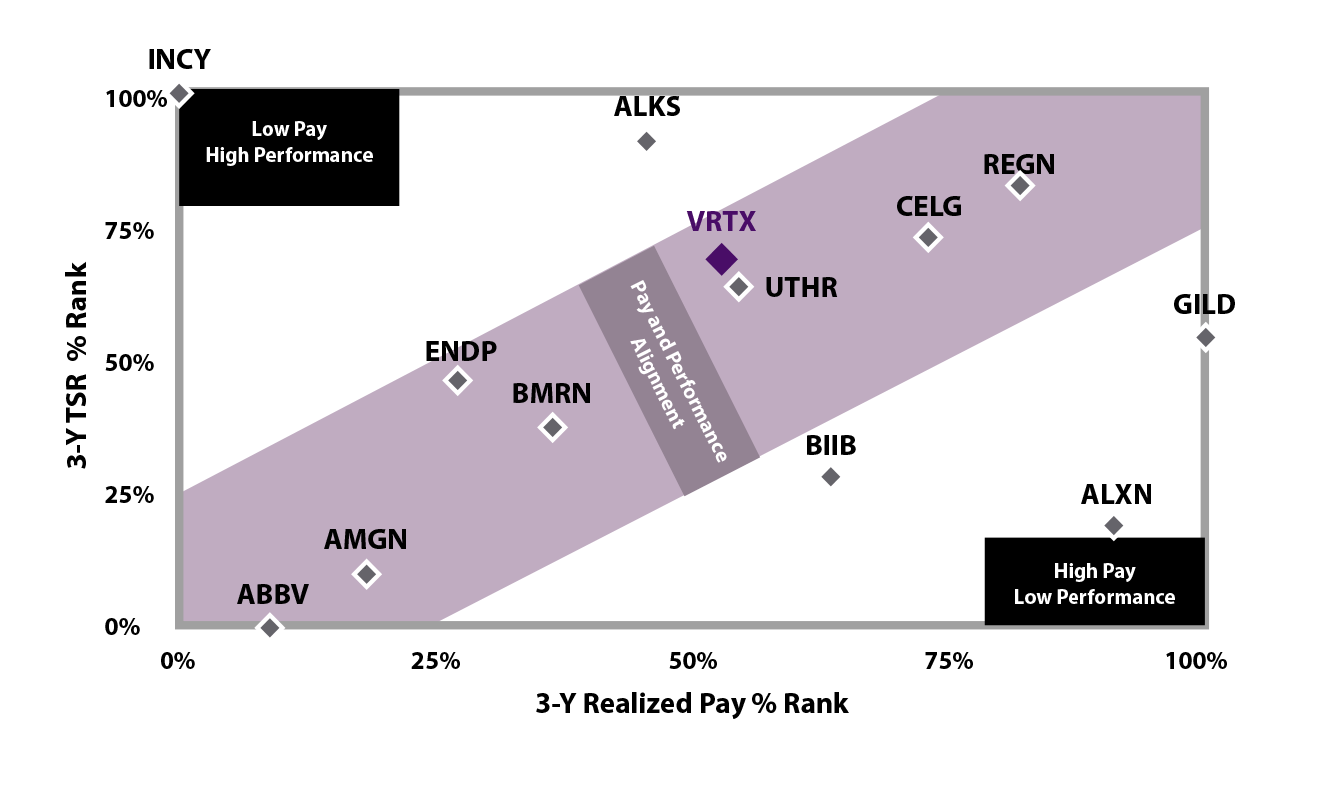
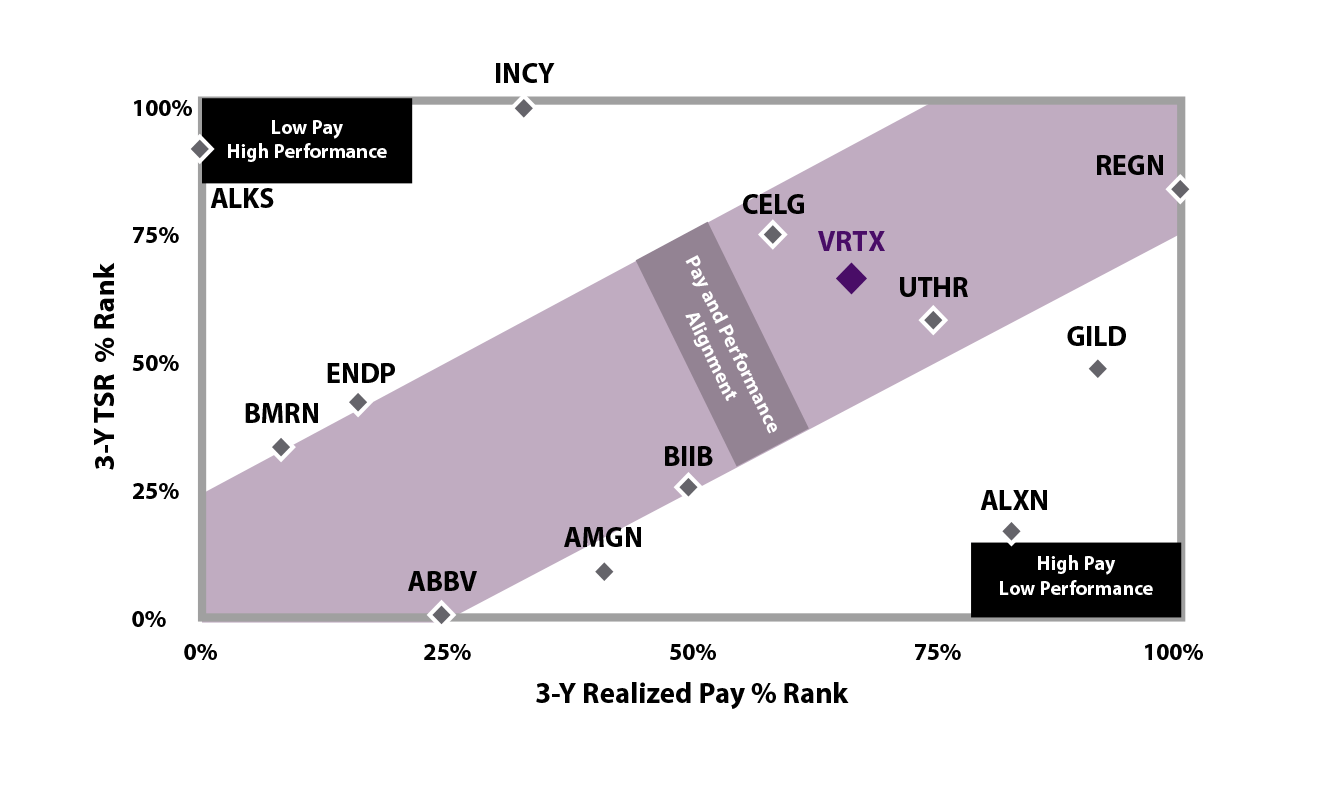
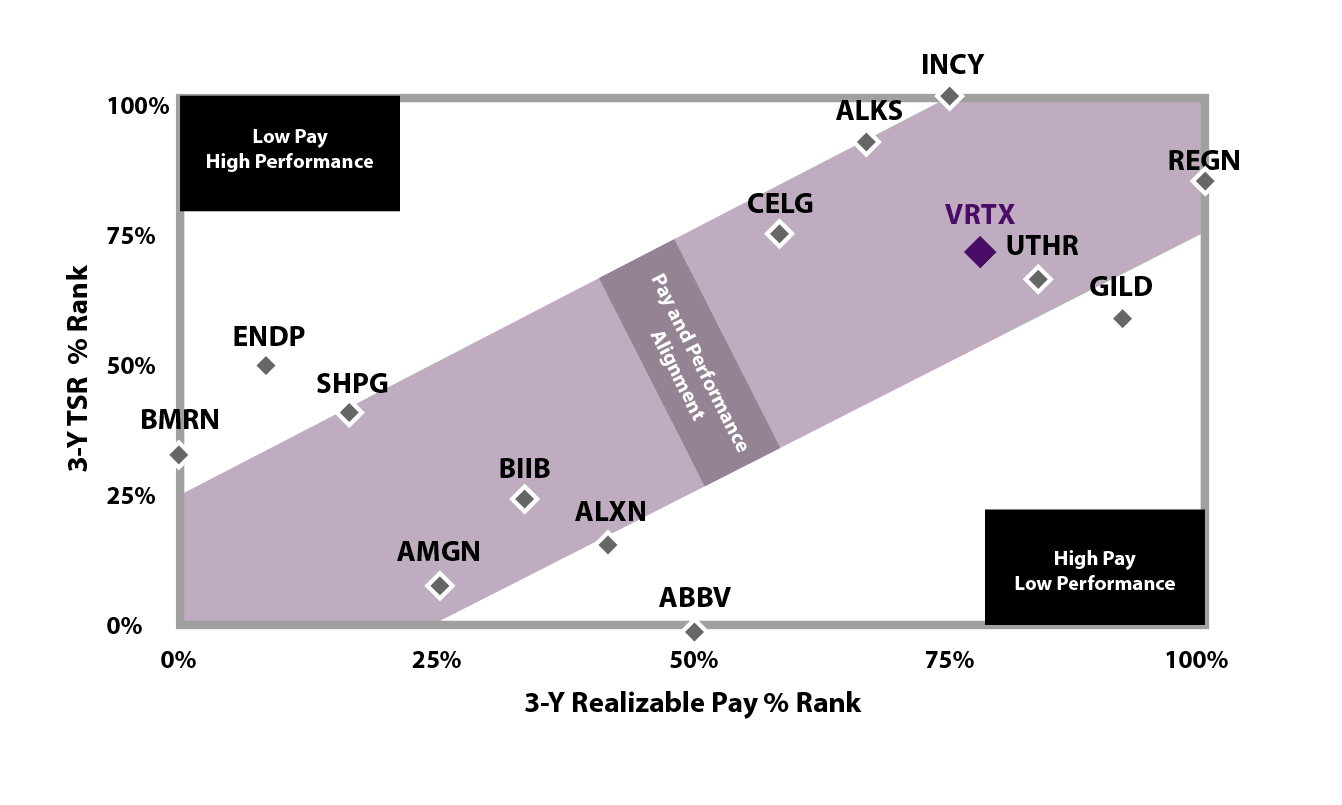
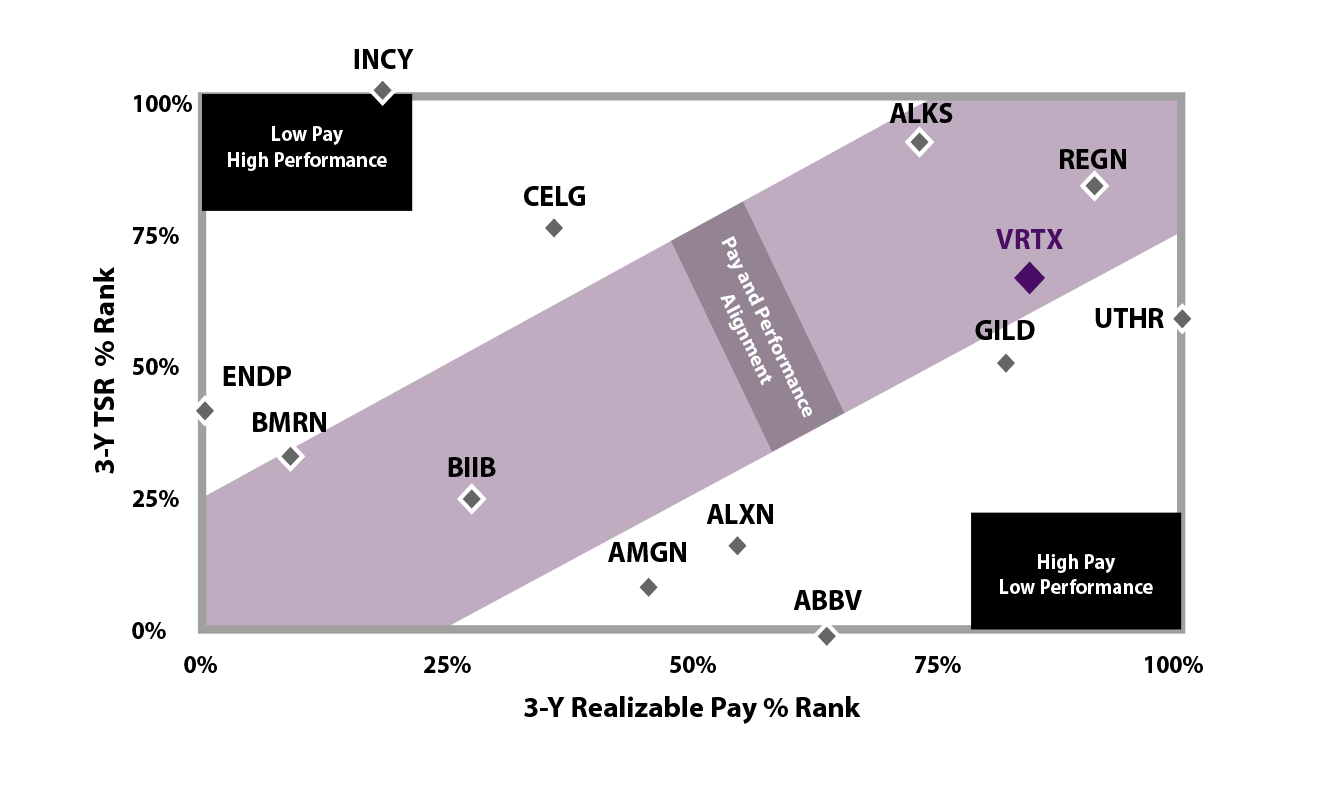
Since our inception, we have compensated all eligible employees using a mix of cash and equity. The broad-based nature of our equity compensation program is an important element of our overall employee compensation program and reflects our philosophy that it is important for all of our employees to approach their jobs with a long-term commitment and perspective. Over the last several years, we have modified our equity compensation programs. These modifications are consistent with modifications other biotechnology companies have made as they matured from development-stage companies to commercial-stage companies with a strong financial profile. As a result of these changes, we granted, on an absolute basis, equity awards representing 33%41% fewer shares of common stock in 20152023 as compared to 20122019 and reduced our "burn rate"“gross burn rate” to 0.8% in 2023 from 3.6%1.4% in 2012 to 2.1% in 2015.
| 2019 | 2020 | 2021 | 2022 | 2023 | % Change 2019 to 2023 | |||||||||||||||||||
| (in thousands, except percentages and employee numbers) | ||||||||||||||||||||||||
| Total Shares Granted Subject to Equity Awards | 3,687 | 1,814 | 2,908 | 2,338 | 2,171 | (41 | )% | |||||||||||||||||
| Gross Burn Rate(1) | 1.4 | % | 0.7 | % | 1.1 | % | 0.9 | % | 0.8 | % | ||||||||||||||
| Awards Canceled, Forfeited or Expired | 886 | 432 | 646 | 311 | 245 | |||||||||||||||||||
| Net Dilution | 2,801 | 1,382 | 2,262 | 2,027 | 1,926 | (31 | )% | |||||||||||||||||
| Net Burn Rate | 1.1 | % | 0.5 | % | 0.9 | % | 0.8 | % | 0.7 | % | ||||||||||||||
| Average # of Employees During Fiscal Year(2) | 2,668 | 3,113 | 3,655 | 4,280 | 5,004 | |||||||||||||||||||
| (1) | Burn rate” is defined as the number of equity awards granted in a specific year divided by the basic weighted average number of shares outstanding during that year. | |
| (2) | ||
| 2012 Equity Awards | 2013 Equity Awards | 2014 Equity Awards | 2015 Equity Awards | % Change 2012 v 2015 | ||||
| Total Shares Subject to Equity Awards | 7,525,000 | 6,276,000 | 5,629,000 | 5,035,000 | (33)% | |||
| Burn Rate (1) | 3.6% | 2.8% | 2.4% | 2.1% | ||||
The information set forth belowamounts for 2015 NEO compensation reflects compensation received under our prior executive compensation program, which we revised in early 2016. Total compensation for our NEOs in 2015 is set forth under the caption “Total Compensation” in the table below. To supplement this information, we have included a column entitled “Total Realized Compensation,” which subtracts the grant-date fair value of equity awards granted in 2015 and substitutes the actual value realized on the exercise of stock options and the vesting of restricted stock awards during 2015.
Named Executive Officer | Salary | Annual Cash Bonus | Grant-Date Fair Equity Awards | Total Compensation | Total Realized Compensation | ||||||||||||||||
| Jeffrey M. Leiden | $ | 1,297,692 | $ | 3,463,200 | $ | 23,325,824 | $ | 28,099,826 | $ | 12,513,357 | |||||||||||
| Ian F. Smith | $ | 701,796 | $ | 832,500 | $ | 7,458,577 | $ | 9,005,983 | $ | 4,837,625 | |||||||||||
| David Altshuler | $ | 528,846 | $ | 552,628 | $ | 11,043,284 | $ | 12,387,868 | $ | 2,574,284 | |||||||||||
| Stuart A. Arbuckle | $ | 629,262 | $ | 721,500 | $ | 7,458,577 | $ | 8,822,449 | $ | 11,961,471 | |||||||||||
| Jeffrey Chodakewitz | $ | 615,231 | $ | 617,382 | $ | 6,582,549 | $ | 7,830,416 | $ | 2,355,296 | |||||||||||
| Company Information | R&D Expense (1) | Operational Focus | Innovative and Importance of Medicines | Market Position | |||||||||
| Company | Industry | $ (millions) | % of Revenue | Global | Commercial | Orphan/Unmet Clinical Need | Breakthrough Therapy Designations | Innovative Drugs in Last 5 Years (2) | Uses Vertex as Peer | Nasdaq 100 | S&P 500 | ||
| AbbVie | Biotech | $ | 4,101 | 18% | √ | √ | √ | 1 | 2 | √ | |||
| Alexion | Biotech | $ | 709 | 27% | √ | √ | √ | 1 | 2 | √ | √ | √ | |
| Alkermes | Biotech | $ | 336 | 54% | √ | √ | √ | 0 | 1 | √ | |||
| Amgen | Biotech | $ | 4,006 | 19% | √ | √ | √ | 1 | 3 | √ | √ | ||
| Biogen | Biotech | $ | 2,012 | 22% | √ | √ | √ | 0 | 2 | √ | √ | √ | |
| BioMarin | Biotech | $ | 635 | 71% | √ | √ | √ | 0 | 1 | √ | √ | ||
| Celgene | Biotech | $ | 2,090 | 23% | √ | √ | √ | 0 | 3 | √ | √ | ||
| Endo | Pharma | $ | 102 | 3% | √ | √ | 0 | 0 | √ | √ | √ | ||
| Gilead | Biotech | $ | 3,014 | 9% | √ | √ | √ | 3 | 4 | √ | √ | √ | |
| Incyte | Biotech | $ | 481 | 64% | √ | √ | 0 | 1 | √ | √ | |||
| Regeneron | Biotech | $ | 1,621 | 40% | √ | √ | √ | 1 | 4 | √ | √ | √ | |
| Shire | Pharma | $ | 920 | 14% | √ | √ | √ | 0 | 3 | ||||
| United Therapeutics | Biotech | $ | 245 | 17% | √ | √ | √ | 0 | 3 | √ | |||
| Vertex | Biotech | $ | 996 | 96% | √ | √ | √ | 4 | 3 | √ | √ | ||
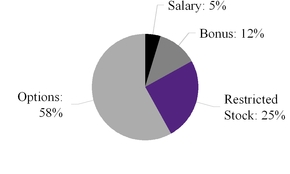
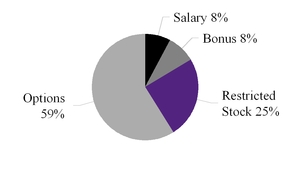
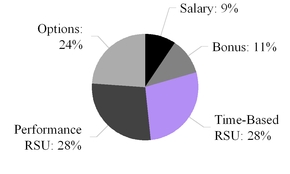
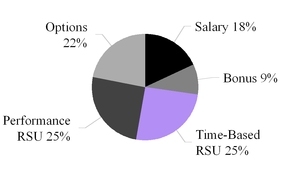
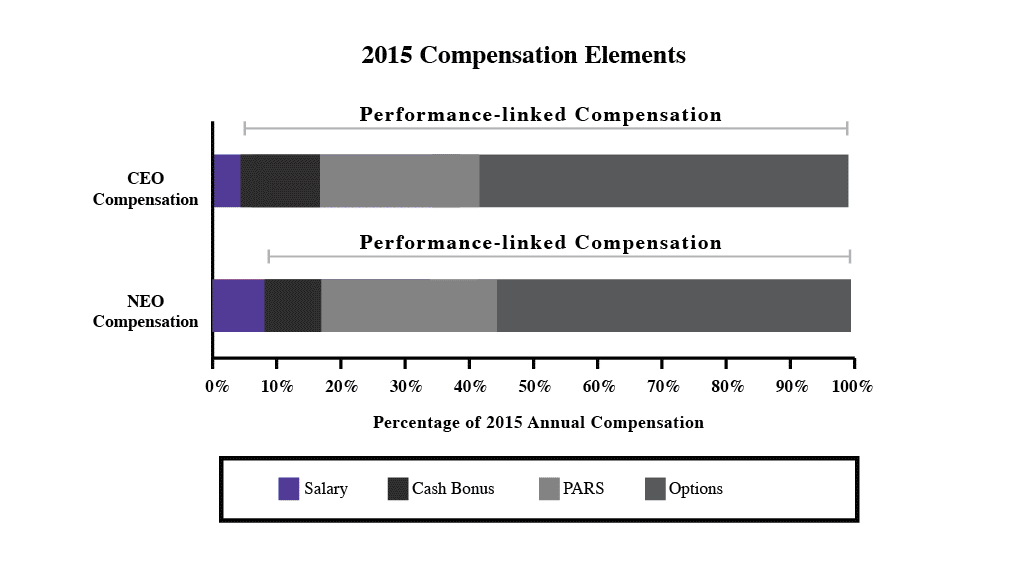
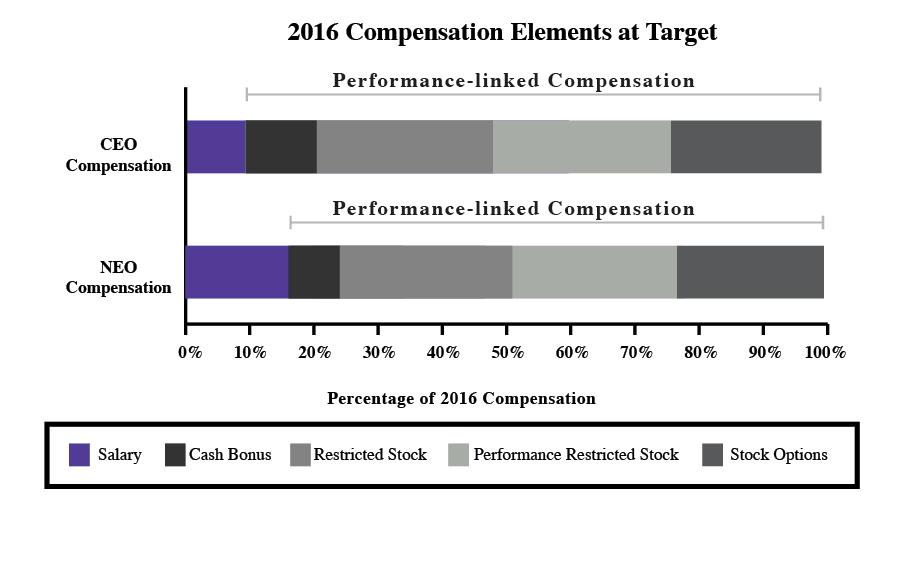
| Name | 2014 Base Salary | % of Peer Group | 2015 Base Salary | % of Peer Group | % Change 2014 v 2015 |
| Jeffrey M. Leiden | $1,100,000 | 35th | $1,300,000 | 50th | 18% |
| Ian F. Smith | $650,000 | 35th | $750,000 | 50th | 15% |
| David Altshuler | na | na | $550,000 | 55th | na |
| Stuart A. Arbuckle | $600,000 | 40th | $650,000 | 50th | 8% |
| Jeffrey Chodakewitz | $600,000 | 60th | $618,000 | 60th | 3% |
At the beginning of each year, our MDCC and board, of directors, in consultation with our CEO, establishesestablish company-wide goals for that year. OurWhile our performance against these goals is the most important factor considered by the board in assessing our corporate performance, but our MDCC and board considersconsider additional accomplishments and shortcomings and may increase or decrease the performance scores. Althoughscores associated with these goals. The aggregate discretionary adjustment may not exceed 10% of the directorsperformance score and the total company score may not exceed 150. The MDCC and our board discuss and analyze ourthe company’s performance, as a group, each director makes his or her own judgment aboutincluding specific performance factors and accomplishment of company goals, and ultimately approve the goals in reaching a conclusion.
For
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 62
Our
| Goal(s) | Maximum Score | Actual 2015 Performance Score |
| Marketed and Approval-Stage Products | 60 | 60 |
| • Expand KALYDECO label in U.S. and ex-U.S. markets and support adherence for KALYDECO patients through compliant marketing practices | ||
| • Support activities to obtain marketing approval for ORKAMBI in the U.S. by mid-2015 and in the European Union by end of 2015 | ||
| • Prepare for and launch ORKAMBI in U.S. and European Union pending regulatory approvals | ||
| Pipeline Growth | 45 | 38 |
| • Advance next-generation CFTR correctors into clinical development | ||
• Advance Phase 3 clinical trials of VX-661 | ||
| • Maintain high productivity in research and early-stage development to expand pipeline | ||
| • Execute collaborations to support and diversify the pipeline and monetize non-core pipeline assets | ||
| Organizational Development and Capability | 15 | 15 |
| • Attract, develop and retain Vertex expertise and key talent necessary to drive near- and long-term company growth | ||
| • Continue to implement our international expansion strategy | ||
| • Support U.S. and international efforts to support access to ORKAMBI | ||
| • Continue our leadership and commitment to the global CF community | ||
| • Continue to ensure a strong compliance mindset and enterprise-wide risk management program | ||
| Financial Strength | 30 | 30 |
| • Manage balance sheet to sustain financial capacity for future investment | ||
| Additional Factors (see page 57 of this proxy statement) | 5 | |
| Total | 150 | 148 |
| Goal(s) | Maximum Score | Actual 2023 Performance Score | |||
| Marketed and Late-Stage Products | 50 | 49.5 | |||
 | Achieve CF net product revenue goals through compliant marketing practices, including U.S. and ex-U.S. revenue goals | ||||
 | Complete key launch readiness activities to support broad patient access to CASGEVY | ||||
 | Establish market strategy for acute pain in the U.S. and complete key hiring to support launch | ||||
 | Advance disease awareness efforts for AMKD in the U.S. | ||||
| Pipeline Growth | 59 | 55.5 | |||
 | Complete Phase 3 trial for the vanzacaftor triple | ||||
 | Obtain marketing approval for CASGEVY | ||||
 | Advance CF mRNA therapy program | ||||
 | Advance multiple non-CF development programs, including completion of the VX-548 pivotal trial in acute pain | ||||
 | Advance multiple research programs, including CF and non-CF programs | ||||
| Manufacturing, Innovation, Quality & Operations | 13 | 13 | |||
 | Complete various commercial manufacturing readiness processes for CASGEVY launch | ||||
 | Continue to enhance supply chain resiliency | ||||
 | Advance manufacturing plans and capabilities for pipeline products | ||||
| Organizational Development and Capability | 13 | 12.5 | |||
 | Continue to build the organization, expand capabilities, and foster an inclusive and equitable culture | ||||
 | Continue to enhance enterprise risk management efforts to match increasing scale and complexity | ||||
 | Advance data strategy | ||||
 | Enhance communications and outreach to support business progression | ||||
| Financial Strength | 15 | 15 | |||
 | Continue to manage our financial resources and to achieve financial targets | ||||
| Additional Accomplishments and Shortcomings, Net (see page 65 of this proxy statement) | 4 | ||||
| TOTAL (FINAL SCORING ROUNDED TO WHOLE NUMBER) | 150 | 150 | |||
Our
Goals - Marketed and Approval-StageLate-Stage Products
In 2023, we made significant progress with respect to $631.7 million in 2015. The increase was due to additional patients being treated with KALYDECO as we completed reimbursement discussions in various jurisdictions,our marketed and increased the number of patients eligible to receive KALYDECO through label expansions and the maintenance of high compliance rates.
 | CF net product revenues increased to $9.87 billion, up 11% as compared to 2022. Our CF net product revenues exceeded the mid-point of our initial CF net product revenues guidance by $244.2 million ($9.87 billion actual as compared to the mid-point of our initial guidance of $9.63 billion) as a result of continued strong uptake of TRIKAFTA/KAFTRIO in ex-U.S. markets and the continued performance of TRIKAFTA in the U.S., following the launch of TRIKAFTA in children with CF 2 to 5 years of age. |
 | We completed key cross-functional commercial readiness for the launch of CASGEVY, including with respect to onboarding authorized treatment centers, and continued progress with policymakers, payers and patient advocacy groups. |
 | Developed and completed innovative go-to-market model and launch plan for acute pain in the U.S. and made significant progress in building our pain organization with key critical hires. |
 | Increased AMKD disease awareness and diagnosis through awareness and testing campaigns. |
For marketed and approval-stageapproval- or late-stage products goals, our board assigned the company a score of 6049.5 out of 60,50, due to (1) our success in expanding our KALYDECO revenues, which resulted in KALYDECOexceeding goals with respect to total CF net product revenues, that increased by 36%successfully achieving launch readiness for CASGEVY, and (2) our achievementsmaking meaningful progress in obtaining approval for ORKAMBI in the U.S. and E.U, and commercializing ORKAMBI in the U.S. and preparing for the global launch of ORKAMBI.non-CF late-stage products.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 63
Goals - Pipeline Growth (Late and Early-Stage)
In 2015,2023, we built onmade significant progress advancing our leadership position in the treatmentpipeline of CF and advancing and broadening our pipeline. Specifically, we:
 | We progressed Phase 3 development of the vanzacaftor triple to completion. |
 | |
 | We advanced our CF mRNA program, VX-522, designed for people not currently eligible for any of |
| the clinical trial. | |
 |
 | We expanded the eligibility of our CF medicines to |
 | We made significant progress advancing a broad pipeline of potentially transformative small molecule, cell and genetic therapies aimed at treating serious diseases. |
 | We completed enrollment in the Phase 2B dose-ranging portion of the study of inaxaplin, our small molecule inhibitor of APOL1 function for people with AMKD. |
 | We completed enrollment in Part C of the Phase 1/2 clinical |
 | We initiated a Phase 1/2 clinical trial evaluating VX-670 for people with DM1. |
On the basis of the accomplishments in advancing our research and developmentR&D programs and, in particular the regulatory submissions and multiple approvals for CASGEVY, and the advancement of multiple pre-clinical candidates across multiple modalities, our board assigned the company a score of 55.5 out of 59 for our pipeline growth goal.
Goals - Manufacturing, Innovation, Quality and Operations
 | We advanced various commercial manufacturing readiness processes for the launch of CASGEVY. |
 | We ensured the continued uninterrupted supply of our CF medicines by meeting stock targets, advancing commercial supply plans for critical materials, and achieving successful inspection results. |
 | We advanced our commercial manufacturing strategy and made advancements in our commercial manufacturing processes for pipeline products. |
In recognition of achieving manufacturing milestones, including ensuring commercial manufacturing launch readiness for CASGEVY, advancing the commercial manufacturing strategy for pipeline programs and strengthening of our CF programs across multiple initiatives including research and business development, partially offset by delays with respect to certain activities,supply chain, the board assigned the company a score of 3813 out of
Goals - Organizational Development and Capability
 | With an emphasis on sustaining our values and culture of innovation, we continued to foster an inclusive and equitable culture that enables employees with different backgrounds to thrive, with particular focus on attracting and retaining a diverse employee base. |
 | Throughout 2023, we maintained above-industry benchmark employee satisfaction and sense of belonging as measured through our bi-annual pulse surveys. |
 | We completed all planned critical hires and succession planning for all senior executives, and maintained low voluntary attrition. |
 | We continued to enhance our risk management. |
 | We continued to prioritize our technology infrastructure to support the organization. |
 | We continued to enhance our corporate reputation and product communications. |
To reflect the improvements to our organizational structure, processes, and systems achieved in 2015,2023, our board assigned the company a score of 12.5 out of 13 for our organizational development and capability goals.
Goals - Financial Strength
We exceeded our financial goals in 2023. We reached our target adjusted non-GAAP EBITDA, and surpassed our target adjusted non-GAAP net income.
As a result of our strong financial performance, including reaching our target adjusted non-GAAP EBITDA and surpassing our target adjusted non-GAAP net income, our board assigned the company a score of 15 out of
15VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 64
Additional Factors
In connection with determining our 20152023 company rating, our board of directors made a net positive five point adjustmentand negative adjustments based on additional factors that were not anticipated in ourwhen the company’s original goals including positive factors such asfor 2023 were established. By design, potential adjustments are capped at ±10% (or ±15 points) and used by the board to address and highlight important achievements and shortcomings. Positive adjustments were related to advancing additional novel, non-CF molecules and therapies, beyond what was planned, and exemplary execution of CASGEVY regulatory submissions and launch planning resulting in multiple breakthrough designations and approvals across multiple regions of the first CRISPR-based gene-edited cell therapy. Overall, the board of directors increased our regulatory strategycompany rating by five points for KALYDECO label expansion, our overall financial performance, advancement of VX-970 through engagement with the National Cancer Institute and implementation of initiatives to foster innovation, partiallythese positive additional accomplishments, which was offset by certain negative factorsa one point reduction related to our operations, including the slower than expected enrollmentplanned progression in one program. As a result, our final company rating was increased by four points to a total of certain clinical trials.
The MDCC evaluates executives’each executive’s individual performance on a “results-based, values-tempered” basis, which takes into account not only “what” was accomplished, but “how” it was accomplished. The results-based component evaluates the executive officer’s performance in his or hertheir individual role and as a leader of our company in achieving our objectives. The possible individual results-based performance ratings are “not building,” “building,” “strong”“strong,” or “leading.” The values-tempered component of the individual evaluations builds upon our company core values: “uncompromising commitment to patients;” “innovation is our lifeblood;” “fearless pursuit of excellence” and “we wins” and are based on whether the decisions made by the executives were consistentconsidered along with theseour leadership competencies, which reflect our core values and what is in the best interestsleadership behaviors that we believe lead to successful execution of the company in the long term. Under our Values Into Practice program, westrategy and continued emphasis on innovation and collaboration. We expect all employees to demonstrate our company core values and leadership behaviors in all aspects of job performance. We further expect that our executives will be stewards of our core values,company culture, and the performance ratings assigned to them incorporate our MDCC and board’s assessment of the strength of their leadership with respect to, and demonstration of, values-based behavior. This evaluation results in ratings of “not demonstrating,“inconsistent demonstration,” “living the values”values,” or “exemplary demonstration.” The possible individual performance ratings under this program are as set forth in the following table:
The
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 65
to the executive’s daily interactions in carrying out his or hertheir duties. Furthermore, the MDCC believes that, in hisher role as CEO, Dr. LeidenKewalramani had greater
In light of Dr. Leiden’s employment agreement, which reduced his base salary and bonus to zero following his first year as Executive Chairman, the MDCC did not assign an individual performance rating for Dr. Leiden.
| Dr. Reshma Kewalramani | 2023 Rating: | Leading Exemplary | |||||
| CEO and President | 2023 Salary: | $ | 1,500,000 | ||||
| 2023 Bonus: | $ | 4,050,000 | |||||
| LTI Equity Grants (Feb 2024): | $ | 17,145,000 | |||||
On the basis of the MDCC’s recommendation, our independent directors rated Dr. Leiden’sKewalramani’s overall performance for 20152023 as “leading/exemplary”“Leading Exemplary,” with an individual performance factor of 150%. The performance rating for Dr. LeidenKewalramani combined a “leading” results-based rating with an “exemplary demonstration” values-basedbehaviors-based rating. TheDr. Kewalramani’s rating derived principally from hisher leadership of our executive team as we executed our strategy for 2015, which was highlighted by:
 | Exemplary leadership over all aspects of the company, including research, development, manufacturing, commercial, and financial, as well as in executing our corporate strategy to develop transformative medicines for serious diseases and achieving our business goals, including recent approvals for CASGEVY, the first-ever CRISPR-based gene-edited cell therapy approved in the world, and which brings a potential functional cure to people with SCD or TDT across multiple regions |
 | Exhibiting outstanding leadership qualities in scaling the organization, advancing Vertex’s culture and values, and recruiting, retaining and developing top-tier talent across the organization, including identifying and developing senior-level talent |
 | Continued execution in growing, transforming, and diversifying our pipeline and commercial medicines into multiple new disease areas, utilizing multiple therapeutic modalities with increasing complexity, while ensuring that quality and compliance remain paramount |
 | The over-achievement of our financial goals, including significantly increasing CF net product revenues, strengthening our balance sheet, and continued delivery of strong operating margins |
 | Leadership and oversight of the advancement of the CF, pain, AMKD, T1D, and DM1 programs, as well as our preclinical pipeline |
 | Building an excellent relationship with the board based on trust, transparency, clear communication and responsiveness |
| Charles F. Wagner, Jr. | 2023 Rating: | Leading Exemplary | |||||
| EVP, Chief Financial Officer | 2023 Salary: | $ | 825,000 | ||||
| 2023 Bonus: | $ | 1,256,063 | |||||
| LTI Equity Grants (Feb 2024): | $ | 6,000,000 | |||||
The MDCC recommended a “leading/exemplary”and the board adopted an overall rating of “Leading Exemplary” for Mr. SmithWagner based on a results-based rating of “leading” and a values-basedbehaviors-based rating of “exemplary demonstration” with an individual performance factor of 145%. Mr. Wagner’s rating derived from his leadership of the finance, accounting, investor relations, and facilities and real estate functions, including the following:
 | Overseeing an outstanding financial year for Vertex, including managing operating expenses in accordance with our budget and guidance |
 | Successfully managing our capital allocation, including execution of a new share repurchase program |
 | Exemplary leadership of the finance, accounting, investor relations, business development, and facilities organizations, including maintaining a high level of shareholder engagement, and significant progress with respect to the construction of new facilities, and successfully recruiting, developing, and mentoring key talent |
 | Leading the successful integration of ViaCyte and strategic transactions including the acquisition of the novel G protein-coupled receptor program from Septerna, Inc., and advanced our strategic collaborations with Entrada and CRISPR Therapeutics |
 | Continuing to improve processes while focusing on areas that increase operational efficiencies, agility, and mitigate risk |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 66
| David M. Altshuler | 2023 Rating: | Leading Exemplary | |||||
| EVP, Chief Scientific Officer | 2023 Salary: | $ | 825,000 | ||||
| 2023 Bonus: | $ | 1,299,375 | |||||
| LTI Equity Grants (Feb 2024): | $ | 6,000,000 | |||||
The MDCC recommended and the board adopted an overall rating of “Leading Exemplary” for Dr. Altshuler based on a results-based rating of “leading” and a behaviors-based rating of “exemplary demonstration” with an individual performance factor of 150%. Dr. Altshuler’s rating derived from his leadership of the global research, preclinical sciences, external innovation, data science, and data technology and engineering organizations, including the following:
 | Overall advancement of a broad pipeline of potentially transformative treatments, including small molecule, oligonucleotide, cell and genetic therapies aimed at treating serious diseases, including rapid progress from bench to clinic of multiple assets, such as DM1 and multiple follow-on molecules in existing diseases areas |
 | Leadership of the scientific aspects of the company’s preparations for, and presentations during, the FDA Advisory Committee meeting regarding CASGEVY |
 | Scientific oversight of the external innovation function and in advancing multiple business development opportunities |
 | Transforming the data science and the data, technology and engineering organizations, driving operational excellence, with a focus on information security, advanced analytics and innovative data solutions |
| Stuart A. Arbuckle | 2023 Rating: | Leading Exemplary | |||||
| EVP, Chief Operating Officer | 2023 Salary: | $ | 900,000 | ||||
| 2023 Bonus: | $ | 1,822,500 | |||||
| LTI Equity Grants (Feb 2024): | $ | 7,125,000 | |||||
The MDCC recommended and the board adopted an overall rating of “Leading Exemplary” for Mr. Arbuckle based on a results-based rating of “leading” and a behaviors-based rating of “exemplary demonstration” with an individual performance factor of 150%. Mr. Smith’s rating was due to his overall contributions to the execution of our strategy with a focus on performance of the finance/accounting, business development, information systems and investor relations functions. More specifically, Mr. Smith was responsible for:
 | Delivering net product revenues of $9.87 billion in 2023, an increase of 11% compared to 2022, and exceeding our initial revenue forecast by $244.2 million |
 |
| TDT, reimbursement agreements, and continued and rapid uptake in eligible people | |
 | |
 | Leadership of the activities preparing for the |
 |
The 2023 cash bonus for each NEO (referred to in the
Summary Compensation Tableon page| Target Cash Bonus |  | Performance Factors |  | Cash Bonus | ||||||||||||
| Base Salary |  | Individual Incentive Target (expressed as a percentage of base |  | Company Performance Factor (expressed as a percentage of target bonus) |  | Individual Performance Factor (expressed as a percentage of the target bonus) |  | Annual Cash Bonus Award | ||||||||
| 70%-120% based on role | 0% | 0-150% | ||||||||||||||
The individual incentive targets were established, and are reviewed annually, by the MDCC based on available data about Peer Group company compensation. Thesecompensation (as supplemented for Mr. Arbuckle, as described above). For 2023, Dr. Kewalramani’s individual incentive targets havetarget remained unchanged since we modified ourat 120% of her base salary and Mr. Arbuckle’s individual incentive target remained at 90% of his base salary. The individual incentive target for Mr. Wagner and Dr. Altshuler remained at 70% of their respective base salary during 2023; Dr. Leiden does not receive an annual cash compensation program in 2012.bonus pursuant to his employment agreement. The resulting target annual cash bonuses of our executives approximate the median target annual cash bonuses for comparable executives at companies in our Peer Group.Group companies (as supplemented for Mr. Arbuckle, as described above).
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 67
Company performance factors are determined annually and range from 0% to 150%. The possible individual ratings and corresponding individual performance factor ranges for our executive officers in 20152023 are set forth in the table below:
| Individual Rating | Individual Performance Factor |
| Not Building | 0% |
| Building | 50%-80% |
| Strong | 80%-120% |
| Leading | 120%-150% |
| Leading/Exemplary |
On the basis of the factors described above, our MDCC recommended, and our independent directors approved, upon the MDCC’s recommendation, individual performance factors and annual bonus awards for each of the NEONEOs, on account of 20152023 performance, as set forth in the table below.
| Name | 2015 Target Bonus | Company Performance Factor | Individual Performance Factor | Proration Factor | 2015 Performance Cash Bonus | ||||||||
| Jeffrey M. Leiden | $ | 1,560,000 | x | 148% | x | 150% | x | 100% | = | $ | 3,463,200 | ||
| Ian F. Smith | $ | 375,000 | x | 148% | x | 150% | x | 100% | = | $ | 832,500 | ||
| David Altshuler | $ | 275,000 | x | 148% | x | 140% | x | 97% | = | $ | 552,628 | ||
| Stuart A. Arbuckle | $ | 325,000 | x | 148% | x | 150% | x | 100% | = | $ | 721,500 | ||
| Jeffrey Chodakewitz | $ | 309,000 | x | 148% | x | 135% | x | 100% | = | $ | 617,382 | ||
| Name | 2023 Base Salary | Individual Incentive Target | 2023 Target Bonus | Company Performance Factor | Individual Performance Factor | 2023 Performance Cash Bonus | ||||||||||||||||||||||
| Reshma Kewalramani | $ | 1,500,000 | x | 120% | = | $ | 1,800,000 | x | 150% | x | 150% | = | $ | 4,050,000 | ||||||||||||||
| Charles F. Wagner, Jr. | $ | 825,000 | x | 70% | = | $ | 577,500 | x | 150% | x | 145% | = | $ | 1,256,063 | ||||||||||||||
| David M. Altshuler | $ | 825,000 | x | 70% | = | $ | 577,500 | x | 150% | x | 150% | = | $ | 1,299,375 | ||||||||||||||
| Stuart A. Arbuckle | $ | 900,000 | x | 90% | = | $ | 810,000 | x | 150% | x | 150% | = | $ | 1,822,500 | ||||||||||||||
| Jeffrey M. Leiden | $ | — | x | —% | = | $ | — | x | —% | x | —% | = | $ | — | ||||||||||||||
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 60
| Back of Contents | ||||
Under our compensation program, equity awards grantedfor our CEO and EVPs are calculated by multiplying the individual’s target equity award by his or her performance rating-based equity modifier. In 2023, we replaced the performance-specific multiplier with a performance-specific multiplier range, as outlined below. Prior to the 2023 performance year, our namedcompensation program provided a specific equity modifier for each performance rating. Our new practice increases the performance sensitivity of the program by adding the flexibility to adjust the equity modifier to reflect performance and expected contributions, further aligning the interests of our CEO and EVPs with the interests of our shareholders.
The target value, available individual ratings and corresponding performance rating-based equity modifier ranges for our executive officers are set forth in the table below.
| Performance Rating-Based Equity Modifiers | |||||||||||||
| Target value | Not Building | Building | Strong | Leading | Leading Exemplary | ||||||||
| CEO | $ | 13,500,000 | —% | 50% - 86.5% | 86.5% - 113.5% | 100% - 127% | 113.5% - 127% | ||||||
| COO | $ | 4,750,000 | —% | 50% - 80% | 80% - 120% | 100% - 150% | 120% - 150% | ||||||
| EVP (excluding the COO) | $ | 4,000,000 | —% | 50% - 80% | 80% - 120% | 100% - 150% | 120% - 150% | ||||||
The CEO equity modifier range is narrower relative to the COO and EVP ranges in order to reflect the tighter distribution of equity award values among the peer CEO comparators.
Historically, equity awards were determined by the following formulaic approach for each performance rating.
| Performance Ratings | ||||||||||
| Not Building | Building | Strong | Leading | Leading Exemplary | ||||||
| CEO | —% | 50% | 100% | 113.5% | 127% | |||||
| NEOs (excluding the CEO) | —% | 50% | 100% | 125% | 150% | |||||
Based on a comparative analysis of our Peer Group companies, as supplemented to address Mr. Arbuckle’s COO role, the MDCC set a target equity value for Dr. Kewalramani of $13.5 million, and maintained the target equity value for Mr. Arbuckle and our other EVPs of $4.75 million and $4.0 million, respectively. The mid-point of the performance factor range for Leading and highest performance factor for Leading Exemplary performance were selected based on award values approximating the 75th and mid upper quartile percentiles, respectively, of executives at our Peer Group companies. The number of shares granted pursuant to the time-vested RSU award and PSU award was based on the executives' individual rating (i) in 2015 underfair value of our share-based guidelines and (ii) in 2016 under our new value-based guidelines.
| Building | Strong | Leading | Leading and Exemplary | |||||
| Restricted Stock | Stock Options | Restricted Stock | Stock Options | Restricted Stock | Stock Options | Restricted Stock | Stock Options | |
| Chief Executive Officer | 21,500 | 106,500 | 43,000 | 213,000 | 53,750 | 266,250 | 64,500 | 319,500 |
| Executive Vice President | 6,900 | 34,000 | 13,800 | 68,000 | 17,250 | 85,000 | 20,700 | 102,000 |
| Stock Options | Restricted Stock | Value | |||
| Jeffrey M. Leiden | 319,500 | 64,500 | $ | 23,325,824 | |
| Ian F. Smith | 102,000 | 20,700 | $ | 7,458,577 | |
| David Altshuler | 34,000 | 75,000 | $ | 11,043,284 | |
| Stuart A. Arbuckle | 102,000 | 20,700 | $ | 7,458,577 | |
| Jeffrey Chodakewitz | 91,500 | 17,250 | 6,582,549 | ||
| Not Building | Building | Strong | Leading | Leading/Exemplary | |||||||||||
| CEO | $ | — | $ | 5,500,000 | $ | 11,000,000 | $ | 12,500,000 | $ | 14,000,000 | |||||
| EVP | $ | — | $ | 1,500,000 | $ | 3,000,000 | $ | 3,750,000 | $ | 4,500,000 | |||||
In February 2016,2024, upon the MDCC’s recommendation, our independent directors approved upon the MDCC’s recommendation, individual performance factors and 2016 equity awards for 2023 performance for each of the NEOs as set forth in the table below. Dr. Leiden’s equity awards were determined by his employment agreement as described above.
| Name | Individual Performance Rating | Performance Rating-Based Equity Modifier | Performance- Based RSU (50%) | Time-based RSU (50%) | Total Equity Value | ||||||||||
| Reshma Kewalramani | Leading Exemplary | 127% | $ | 8,572,500 | $ | 8,572,500 | $ | 17,145,000 | |||||||
| Charles F. Wagner, Jr. | Leading Exemplary | 150% | $ | 3,000,000 | $ | 3,000,000 | $ | 6,000,000 | |||||||
| David M. Altshuler | Leading Exemplary | 150% | $ | 3,000,000 | $ | 3,000,000 | $ | 6,000,000 | |||||||
| Stuart A. Arbuckle | Leading Exemplary | 150% | $ | 3,562,500 | $ | 3,562,500 | $ | 7,125,000 | |||||||
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 69
| Name | Individual Performance Rating | Performance-Based RSU (35%) | Options (30%) | Time-based RSU (35%) | Total Equity Value |
| Jeffrey M. Leiden | Leading Exemplary | $4,900,000 | $4,200,000 | $4,900,000 | $14,000,000 |
| Ian F. Smith | Leading Exemplary | $1,575,000 | $1,350,000 | $1,575,000 | $4,500,000 |
| David Altshuler | Leading | $1,312,500 | $1,125,000 | $1,312,500 | $3,750,000 |
| Stuart A. Arbuckle | Leading Exemplary | $1,575,000 | $1,350,000 | $1,575,000 | $4,500,000 |
| Jeffrey Chodakewitz | Leading | $1,312,500 | $1,125,000 | $1,312,500 | $3,750,000 |
We annually grant one-year financial-based PSU awards and three-year non-financial based PSU awards. We believe the combination of the one-year financial and three-year non-financial PSUs provides an appropriate balance of near- and long-term incentives for valueour management team. Our near-term objective of 2016 equity-basedgrowing our CF business through increasing the number of people eligible and able to receive our medicines complements our long-term strategic objectives, which require the reinvestment of revenues into R&D to develop additional transformative medicines for serious diseases.
The final performance multipliers for our 2023 financial-based PSU awards were determined by the MDCC and applied to the target units granted to determine the actual units earned and eligible to vest with a payout of 200% in February 2024. The following chart shows the pre-established financial goals and the actual results for the financial-based PSU awards granted in 2023:
| Award | Below Threshold | Threshold | Target | Max | Results | ||||||||||||||||||
| Year | Company Goal | 0% Payout | 50% Payout | 100% Payout | 200% Payout | CF Revenue | Payout | ||||||||||||||||
| 2023 | 2023 CF Net Product Revenues | <$9.400 billion | $ | 9.400 billion | $ $ | 9.550 to 9.650 billion | $ | 9.800 billion | $ 9.86 billion(1) | 200.0% | |||||||||||||
| (1) | Reflects certain pre-established foreign exchange-related adjustments. |
Consistent with our philosophy of aligning compensation with performance, for 2023, a year in which we substantially exceeded our CF net product revenue expectations, the payout on our one-year financial PSU awards achieved the maximum level.
The performance goals for the 2021 non-financial PSUs were established in February 2021 and our performance against these goals was determined in the first quarter of 2024. There were three non-financial goals and achievement of one goal would have resulted in a 50% payout, achievement of two goals resulted in a 100% payout and achievement of three goals would have resulted in a payout of 200%. While we achieved many significant milestones during this period, in part described above, we did not achieve one of the milestones associated with these PSUs.
| Payout | ||||||
| 2021 | CF Portfolio Milestone - Establish proof-of-concept for a Best in Class combination or genetic therapy (excluding the vanzacaftor triple) | Not Achieved | ||||
| Complete two Phase 2 studies in non-cystic fibrosis disease areas, including all non-cystic fibrosis products to the extent not already counted for other proof-of-concept performance milestones and any drug candidates or therapies in-licensed or acquired | Achieved | 100% | ||||
| Obtain U.S. or ex-U.S. regulatory approval (accelerated or full) for a non-cystic fibrosis indication (including any in-licensed drug candidates or therapies) | Achieved |
The final performance multipliers for our 2021 non-financial PSUs were determined by the MDCC and applied to the target units granted to determine the actual units earned and eligible to vest with a payout of 100% in February 2024. Performance achievement for the 2022, 2023 and 2024 non-financial based PSU awards will be determined in the first quarter of 2025, 2026 and 2027, respectively, based on performance over the relevant three-year performance period. The non-financial goals contained in our three-year PSU awards for 2022, 2023 and 2024 are based on our methodology for determining the grant-date fair value, including underlying assumptions for calculating these values as set forth in Note N to our consolidated financial statements included in our 2015 Annual Report on Form 10-Kmultiple clinical milestones, and are subject to adjustment.
Our executives are eligible to participate in all of our benefit plans and programs on the terms made generally available to our employees, including medical insurance, dental insurance, payment of life insurance premiums, disability coverage, equity programs, including a career employment/retirement provision and participation in our employee stock purchase plan.plan, and eligibility for matching contributions, subject to an annual $25,000 limit, to qualified charitable organizations pursuant to the Vertex Foundation Matching Gift Program. We have a defined contribution—a 401(k)—plan, in which all of our eligible employees, including our NEOs, are eligible tomay participate. We make matching contributions to the 401(k) plan. The formula for determining the amount of our matching contributions is the same for our NEOs as for our other employees (and the contributions are subject to the same statutory maximum), but the actual contributions made to the accounts of our NEOs generally are at the top end of the range, due to the executives’ higher salaries and correspondingly higher cash contribution levels. WeOther than the retirement provision under our equity program available to all employees, we do not provide any other retirement benefits to our executive officers. Under his amended employment agreement, Dr. Leiden receives an annual cash payment intended to facilitate participation in the company’s benefit plans.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 70
The initial compensation terms for newly hired members of our executive team are the result of negotiations between us, in consultation with the MDCC and our board of directors, and the executive being recruited. Accordingly, the initial employment terms for each of the executive officers may vary significantly because they take into account both our interests and the executive’s interests under the circumstances at the time of negotiation, and depend on the level of job responsibility, the market for the executive’s services, the value of other opportunities then available to the executive and similar considerations. Executives who join us from other companies may sacrifice potential bonuses and/or equity payouts, and may request compensation elements of similar value. More experienced individuals may seek higher compensation than individuals who are still establishing their careers. We seek to balance the need to be competitive in a competitive market against the need for the executive’s compensation to be comparable with the executive’s peers at the company.hired. In general, each newly hired executive team member enters into an employment agreement and a change of control agreement and is awarded a stock option grant and a restricted stock grant,granted an equity award, and in some cases a cash sign-on bonus, reimbursement of moving expenses, and other benefits. We also enter into employment and change of control agreements with executivesEVPs who are promoted to our executive team, on the basis of standard terms and conditions that have been recommended by our MDCC and approved by our board for such circumstances. We have entered into agreements providing for severance and change of control payments with each of the members ofEVP on our executive team including all of the NEOs, because we believe that they are a fair and effective way to allow our executives to maintain focus on our business in the face of market and other volatility in our industry.
In general, each employment arrangement provides for cash severance and continuation of certain employee benefits in the event that an executive’s employment is terminated by us without cause or is terminated by the executive for good reason. We use a “double trigger” with respect to benefits that are to be provided in connection with a change of control. A change of control does not itself trigger benefits; rather, benefits are paid only if the employment of the executive is terminated by us other than for cause, death or disability, or by the executive for good reason, during a specified period before or after a change of control. We believe a “double trigger” benefit maximizes shareholder value because it prevents a windfall to executives in the event of a change of control in which the executive retains significant responsibility as defined in his or hertheir individual agreement, while still providing our executives appropriate incentives to cooperate in negotiating any change of control transaction that may put their jobs at risk.
We offer a company-wide program that provides for accelerated vesting of equity awards held by qualified retirement-eligible participants that retire. Equity awards granted, including those granted to our NEOs, contain a retirement vesting provision, under which a “qualified” participant who retires under the terms of the provision will receive accelerated vesting of an additional number of shares underlying the award, equal to the sum of (x) 50% plus 10% for each year of service in excess of five full years of service multiplied by (y) the number of unvested shares subject to the award. A “qualified” participant is a participant (1) who is at least age 55, (2) has completed at least five full years of service, (3) whose age plus full years of service is 65 or greater, and (4) who has completed a mandatory transitional period of employment with the company following notice of their planned termination of service.
In addition to the benefits that only accrue in connection with a change of control, our agreements with our executive officers provide benefits if we terminate their employment with us without cause or they terminate their employment with us for good reason, as such terms are defined in the applicable agreement with the executive officer. A further discussion of the terms and projected payments under each of theseour agreements with our NEOs is set forth below under the heading
Under Section 162(m) of the Internal Revenue Code, publicly held corporations generally may not deduct compensation in excess of $1 million paid to certain executive officers, subject to limited transition relief for certain arrangements in place as of November 2, 2017. We would likecontinue to grant performance-based compensation as important elements of our compensation program to be reasonably costthat align corporate shareholder and tax effective. To the extent consistent with our other goals, we seek to preserve corporate tax deductions, while maintaining the flexibility to approve compensation arrangements that we believe are in the bestcompany interests, of the company and our shareholders. The approach doeseven though these awards may not always result in full tax deductibility.
Our board of directors generally grants employee options two times per year, on the date of its mid-summerannual equity awards to NEOs at a board meeting usuallyscheduled in July, and on the date of its first meeting of each new year, usually in late January oradvance for early February. Beginning in 2016, our named executive officers will no longer receive mid-year equity grants. Supplemental equity grants, if any, are made at board meetings at the time when the board determines they are appropriate in order to meet the objectives of our compensation program. Board and committee meetings generally are scheduled at least a year in advance, and schedulingScheduling decisions are made without regard to anticipated earnings or other major announcements by the company.
Newly hired employees, including executive officers, are sometimes granted options and/or restricted stockequity awards effective on the first day of employment, with the options having an exercise price set at the average of the high and low price for our common stock on the employment start date.employment. The employees’ start dates are scheduled without regard to anticipated earnings or other major announcements by the company.
We have adopted a recoupment or claw-backclawback policy providing that is intended to comply with the requirements of the Dodd-Frank Act. Under this policy, in the event we are required to prepare an accounting restatement due to material noncompliance with financial reporting requirements, we are required to recover incentive-based compensation erroneously received by current and former executive officers during the three completed
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 71
fiscal years immediately preceding the year of the restatement. Erroneous payments will be recovered under the policy even if there was no misconduct or failure of oversight on the part of an individual executive officer.
We also have a clawback policy relating to fraud and intentional misconduct. Under that policy, if our board of directors determines that an executive officer engaged in fraud or intentional misconduct that resulted in an incorrect determination that an incentive compensation performance goal had been achieved, the board may take appropriate action to recover from such executive officer any compensation that resulted from such determination. The board may require reimbursement forrepayment of any bonus, equity or incentive compensation awarded to an executive officer who engaged in the fraud or intentional misconduct to the extent it was based on such incorrect determination.
We have stock ownership guidelines for our chief executive officer and NEOs and in 2014 adopted stock ownership guidelines for our non-employee directors, as discussed in
| Employee | |
| Minimum Shareholding Requirement | |
| 6X base salary | |
| Executive | 30% of annual equity grant |
| EVPs | 4X base salary |
Individual holdings, and holdings of immediate family members, of (a) common stock, including(b) unvested restricted stock, (b) restricted stock unitsRSUs, and (c) shares held through our 401(k) plan count toward meeting these guidelines; unearned PSUs and unexercised stock options do not count toward meeting these guidelines. EachAs of March 18, 2024, each of our NEOs including our chief executive officer, currently satisfiessatisfied the individual holding requirements.
Our Insider Trading Policy prohibits all of our directors and employees, including our named executive officers,NEOs, from (i)(a) short selling or hedging our securities, (ii)(b) purchasing or selling derivative securities based on our securities, and (iii)(c) pledging our securities.
Our MDCC reviews the risks and rewards associated with our compensation programs. The programs are designed with features that mitigate risk without diminishing the incentive nature of the compensation. We believe our compensation programs encourage and reward prudent business judgment and appropriate risk-taking over the short term and the long term. Our MDCC regularly evaluates the risks involved with our compensation programs and does not believe that any of our compensation programs create risks that are reasonably likely to have a material adverse effect on our company.
Risk Mitigation Factors
We believe that our annual cash bonus and long-term equity compensation programs, which account for most of our executive officers’ compensation, contain appropriate risk mitigation factors, as summarized below:
Emphasis on Long-term Value Creation and Mitigation of Short-term Risk Taking
Our board believes that a key element of its risk oversight responsibilities is ensuring that our executive compensation program encourages the implementation of our corporate strategy of investing in scientific innovation to create transformative medicines for people with serious diseases and discourages decisions focused on creating short-term financial gains at the expense of long-term value creation. The board reviews our business performance, focusing on financial metrics and non-financial metrics, as well as other strategic factors including talent development and diversity to ensure our leaders are focusing on long-term growth in a manner aligned with our values.
Our MDCC reviews the performance of our executive officers using the above metrics. It also oversees the design of our executive compensation programs to ensure that our executive compensation program does not incentivize our executive officers, either individually or as a group, to make excessively risky business decisions that could maximize short-term results at the expense of long-term value. The independent directors who serve on the MDCC are informed of our most significant risks, including those associated with R&D of new medicines, competition, and the pricing of our medicines. Our MDCC, in consultation with its independent compensation consultant, ensures that our executive compensation programs are aligned with our long-term strategy and do not incentivize overly risky behavior.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 72
| Back of Contents | ||||
The Management Development and Compensation Committee has reviewed the Compensation Discussion and Analysis and discussed that analysis with management. Based on its review and its discussions with management, the Management Development and Compensation Committee recommended to Vertex’s Board of Directors that the Compensation Discussion and Analysis be included in Vertex’s proxy statement for its
Bruce I. Sachs (Chair)
Lloyd Carney
Terrence C. Kearney
Diana McKenzie
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 66
| Back of Contents |
The following table provides summary information concerning the compensation earned byfor each of our chief executive officer; our chief financial officer;NEOs for 2023, 2022, and our other three most highly compensated employees who were serving as executive officers on December 31,
| Name and Principal Position | Year | Salary | Bonus | Stock Awards | Option Awards | Non-Equity Incentive Plan Compensation | All Other Compensation | Total | ||||||||||||||||||||
| Jeffrey M. Leiden | 2015 | $ | 1,297,692 | $ | — | $ | 7,038,885 | $ | 16,286,939 | $ | 3,463,200 | $ | 13,110 | $ | 28,099,826 | |||||||||||||
| Chairman, President & CEO | 2014 | $ | 1,100,000 | $ | — | $ | 19,883,350 | $ | 12,669,261 | $ | 2,970,000 | $ | 12,857 | $ | 36,635,468 | |||||||||||||
| 2013 | $ | 1,038,462 | $ | — | $ | 1,773,963 | $ | 7,529,374 | $ | 2,772,000 | $ | 12,675 | $ | 13,126,474 | ||||||||||||||
| Ian F. Smith | 2015 | $ | 701,796 | $ | — | $ | 2,258,991 | $ | 5,199,586 | $ | 832,500 | $ | 13,110 | $ | 9,005,983 | |||||||||||||
| EVP & Chief Financial Officer | 2014 | $ | 650,000 | $ | — | $ | 9,865,110 | $ | 4,044,646 | $ | 731,250 | $ | 12,857 | $ | 15,303,863 | |||||||||||||
| 2013 | $ | 582,959 | $ | — | $ | 544,988 | $ | 2,361,640 | $ | 682,500 | $ | 12,675 | $ | 4,184,762 | ||||||||||||||
| David Altshuler | 2015 | $ | 528,846 | $ | 250,000 | $ | 9,078,750 | $ | 1,964,534 | $ | 552,628 | $ | 13,110 | $ | 12,387,868 | |||||||||||||
| EVP & Chief Scientific Officer | ||||||||||||||||||||||||||||
| Stuart A. Arbuckle | 2015 | $ | 629,262 | $ | — | $ | 2,258,991 | $ | 5,199,586 | $ | 721,500 | $ | 13,110 | $ | 8,822,449 | |||||||||||||
| EVP & Chief Commercial | 2014 | $ | 600,000 | $ | — | $ | 9,865,110 | $ | 4,044,646 | $ | 675,000 | $ | 12,857 | $ | 15,197,613 | |||||||||||||
| Officer | 2013 | $ | 553,846 | $ | — | $ | 544,988 | $ | 3,077,513 | $ | 630,000 | $ | 12,675 | $ | 4,819,022 | |||||||||||||
| Jeffrey Chodakewitz | 2015 | $ | 615,231 | $ | — | $ | 1,882,493 | $ | 4,700,056 | $ | 617,382 | $ | 15,254 | $ | 7,830,416 | |||||||||||||
| EVP & Chief Medical Officer | 2014 | $ | 539,077 | $ | 250,000 | $ | 8,963,250 | $ | 3,171,829 | $ | 630,000 | $ | 241,936 | $ | 13,796,092 | |||||||||||||
| Name and Principal Position | Year | Salary | Bonus | Stock Awards(1) | Option Awards | Non-Equity Incentive Plan Compensation | All Other Compensation | Total | ||||||||||||||||||||||||
| Reshma Kewalramani | 2023 | $ | 1,500,000 | $ | — | $ | 15,001,894 | $ | — | $ | 4,050,000 | $ | 42,547 | $ | 20,594,441 | |||||||||||||||||
| CEO and President | 2022 | $ | 1,396,154 | $ | — | $ | 10,640,784 | $ | — | $ | 3,784,500 | $ | 43,059 | $ | 15,864,497 | |||||||||||||||||
| 2021 | $ | 1,221,923 | $ | — | $ | 10,924,599 | $ | — | $ | 3,016,570 | $ | 35,764 | $ | 15,198,856 | ||||||||||||||||||
| Charles F. Wagner, Jr. | 2023 | $ | 825,000 | $ | — | $ | 5,250,552 | $ | — | $ | 1,256,063 | $ | 40,136 | $ | 7,371,751 | |||||||||||||||||
| EVP & Chief Financial Officer | 2022 | $ | 793,269 | $ | — | $ | 3,750,197 | $ | — | $ | 1,239,315 | $ | 28,830 | $ | 5,811,611 | |||||||||||||||||
| 2021 | $ | 729,615 | $ | — | $ | 4,375,430 | $ | — | $ | 1,078,539 | $ | 40,348 | $ | 6,223,932 | ||||||||||||||||||
| David M. Altshuler | 2023 | $ | 825,000 | $ | — | $ | 4,375,089 | $ | — | $ | 1,299,375 | $ | 41,636 | $ | 6,541,100 | |||||||||||||||||
| EVP & Chief Scientific Officer | 2022 | $ | 810,577 | $ | — | $ | 4,500,090 | $ | — | $ | 1,172,325 | $ | 42,137 | $ | 6,525,129 | |||||||||||||||||
| 2021 | $ | 756,731 | $ | — | $ | 4,375,430 | $ | — | $ | 1,159,200 | $ | 39,976 | $ | 6,331,337 | ||||||||||||||||||
| Stuart A. Arbuckle | 2023 | $ | 900,000 | $ | — | $ | 6,234,712 | $ | — | $ | 1,822,500 | $ | 41,737 | $ | 8,998,949 | |||||||||||||||||
| EVP & Chief Operating Officer | 2022 | $ | 900,000 | $ | — | $ | 12,294,259 | $ | — | $ | 1,761,750 | $ | 43,097 | $ | 14,999,106 | |||||||||||||||||
| 2021 | $ | 842,308 | $ | — | $ | 5,250,173 | $ | — | $ | 1,676,700 | $ | 40,960 | $ | 7,810,141 | ||||||||||||||||||
| Jeffrey M. Leiden | 2023 | $ | — | $ | — | $ | 6,500,260 | $ | — | $ | — | $ | 95,022 | $ | 6,595,282 | |||||||||||||||||
| Executive Chairman | 2022 | $ | — | $ | — | $ | 8,500,250 | $ | — | $ | — | $ | 92,930 | $ | 8,593,180 | |||||||||||||||||
| 2021 | $ | 242,308 | $ | — | $ | 7,875,259 | $ | — | $ | — | $ | 39,672 | $ | 8,157,239 | ||||||||||||||||||
| (1) | Pursuant to applicable SEC rules, the grant-date fair values of the equity awards granted in February 2023 for 2022 performance are included in 2023 compensation. Also included in 2022 compensation is the grant-date fair value of a one-time equity awards granted in December 2022 to Mr. Arbuckle. The equity awards granted in February 2024 to Dr. Kewalramani, Mr. Wagner, Dr. Altshuler and Mr. Arbuckle for 2023 performance and the equity awards granted in February 2024 to Dr. Leiden pursuant to his employment agreement, in each case that are discussed in the Compensation Discussion and Analysis section above, are not reflected in the Summary Compensation Table above. |
Pursuant to applicable SEC rules, the annual cash bonuses earned by our named executive officersNEOs are set forthincluded under the caption “Non-Equity Incentive Plan Compensation.” Other bonuses, such as sign-on bonuses, are listed separately under the caption “Bonus.”
The amounts set forth under the captionscaption “Stock Awards” and “Option Awards” in the table above represent the grant-date fair value of awards granted during the applicable fiscal year. Our methodologyIn general, the equity awards reflected in the Summary Compensation Table for determininga specific year reflect equity grants made earlier in that calendar year based on the executive’s performance in the year prior to the year the equity grants are awarded. Because a majority of executive compensation is in the form of equity awards, the total compensation reflected in each executive’s compensation for 2023 in the table above is significantly affected by such executive’s performance during 2022.
The “Stock Awards” for 2023, 2022, and 2021 consist of PSU awards and time-vested RSU awards (or, in the case of Dr. Leiden, fully vested common stock) granted in February of each year. In each of 2023, 2022, and 2021, the financial PSU awards had grant-date values of 100% of the fair value of the target shares, respectively, in accordance with U.S. GAAP. In 2023, 2022, and 2021, the non-financial PSU awards had grant-date values of 50%, 0% and 50%, of the fair value of the target shares, respectively, in accordance with U.S. GAAP. Additionally, the special, one-time PSU award granted to Mr. Arbuckle in December 2022 had a grant-date value of 50% of the fair value of the target shares in accordance with U.S. GAAP. For each of these awards, the grant-date fair values were based on the probable outcome of the performance conditions associated with the awards. If the grant-date fair value including underlying estimatesof the financial and assumptions for calculating these values, is set forth in Note
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 67
| Back of Contents |
| Name | 2023 | 2022 | 2021 | ||||||||
| Reshma Kewalramani | $ | 25,717,533 | $ | 21,281,812 | $ | 18,728,038 | |||||
| Charles F. Wagner, Jr. | $ | 9,000,945 | $ | 7,500,393 | $ | 7,500,890 | |||||
| David M. Altshuler | $ | 7,500,153 | $ | 9,000,180 | $ | 7,500,890 | |||||
| Stuart A. Arbuckle | $ | 10,688,305 | $ | 26,488,914 | $ | 9,000,297 | |||||
| Jeffrey M. Leiden | $ | 9,750,389 | $ | 12,750,376 | $ | 13,500,444 | |||||
The amounts set forth under the caption “Non-Equity Incentive Plan Compensation” in the table above represent annual cash bonuses for
| Name | Base Salary | Individual Incentive Target | 2015 Target Bonus | Company Performance Factor | Individual Performance Factor | Proration Factor | 2015 Performance Cash Bonus | ||||||||||||
| Jeffrey M. Leiden | $ | 1,300,000 | x | 120% | = | $ | 1,560,000 | x | 148% | x | 150% | x | 100% | = | $ | 3,463,200 | |||
| Ian F. Smith | $ | 750,000 | x | 50% | = | $ | 375,000 | x | 148% | x | 150% | x | 100% | = | $ | 832,500 | |||
| David Altshuler | $ | 550,000 | x | 50% | = | $ | 275,000 | x | 148% | x | 140% | x | 97% | = | $ | 552,628 | |||
| Stuart A. Arbuckle | $ | 650,000 | x | 50% | = | $ | 325,000 | x | 148% | x | 150% | x | 100% | = | $ | 721,500 | |||
| Jeffrey Chodakewitz | $ | 618,000 | x | 50% | = | $ | 309,000 | x | 148% | x | 135% | x | 100% | = | $ | 617,382 | |||
| Name | Base Salary | Individual Incentive Target | 2023 Target Bonus | Company Performance Factor | Individual Performance Factor | 2023 Performance Cash Bonus | |||||||||||||||||||||
| Reshma Kewalramani | $ | 1,500,000 | x | 120% | = | $ | 1,800,000 | x | 150% | x | 150% | = | $ | 4,050,000 | |||||||||||||
| Charles F. Wagner, Jr. | $ | 825,000 | x | 70% | = | $ | 577,500 | x | 150% | x | 145% | = | $ | 1,256,063 | |||||||||||||
| David M. Altshuler | $ | 825,000 | x | 70% | = | $ | 577,500 | x | 150% | x | 150% | = | $ | 1,299,375 | |||||||||||||
| Stuart A. Arbuckle | $ | 900,000 | x | 90% | = | $ | 810,000 | x | 150% | x | 150% | = | $ | 1,822,500 | |||||||||||||
| Jeffrey M. Leiden | $ | — | x | —% | = | $ | — | x | —% | x | —% | = | $ | — | |||||||||||||
The amounts set forth under the caption “All Other Compensation” in the table for
| Name | 401(k) Match | Life Insurance Premiums | Matching Gift Program | Other | Total | ||||||||||||||
| Reshma Kewalramani | $ | 14,850 | $ | 2,697 | $ | 25,000 | $ | — | $ | 42,547 | |||||||||
| Charles F. Wagner, Jr. | $ | 14,850 | $ | 1,786 | $ | 23,500 | $ | — | $ | 40,136 | |||||||||
| David M. Altshuler | $ | 14,850 | $ | 1,786 | $ | 25,000 | $ | — | $ | 41,636 | |||||||||
| Stuart A. Arbuckle | $ | 14,850 | $ | 1,887 | $ | 25,000 | $ | — | $ | 41,737 | |||||||||
| Jeffrey M. Leiden | $ | 3,015 | $ | 7 | $ | 25,000 | $ | 67,000 | (1) | $ | 95,022 | ||||||||
| (1) | Includes annual cash payment made to Dr. Leiden in order to facilitate his participation in the company’s benefits plans. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 75
The following table provides information with respect to grants of awards to each of our NEOs during 2023. Pursuant to SEC rules, (i) the threshold, target and maximum amounts payable pursuant to our 2023 annual cash bonus program are set forth in columns under “Estimated Possible Payouts under Non-Equity Incentive Plan Awards,” (ii) the threshold, target, and maximum number of shares that could vest pursuant to PSUs granted in 2023 are set forth in columns under “Estimated Future Payouts under Equity Incentive Plan Awards,” and (iii) the number of shares granted pursuant to other RSU awards in 2023 is set forth under “All Other Stock Awards: Number of Shares of Stock or Units.”
| Estimated Possible Payouts Under Non-Equity Incentive Plan Awards | Estimated Future Payouts Under Equity Incentive Plan Awards (shares) | All Other Stock Awards: Number of Shares of | Grant-Date Fair Value of Stock and Option | |||||||||||||||||||||||||||||||
| Name | Grant Date | Threshold ($) | Target ($) | Maximum ($) | Threshold (#) | Target (#) | Maximum (#) | Stock or Units (#) | Awards ($) | |||||||||||||||||||||||||
| Reshma Kewalramani | (1) | $ | — | $ | 1,800,000 | $ | 4,050,000 | |||||||||||||||||||||||||||
| (2a) | 2/1/2023 | — | 13,486 | 26,972 | $ | 4,286,255 | ||||||||||||||||||||||||||||
| (2b) | 2/1/2023 | — | 13,486 | 26,972 | $ | 2,143,128 | ||||||||||||||||||||||||||||
| (3) | 2/1/2023 | 26,972 | $ | 8,572,511 | ||||||||||||||||||||||||||||||
| Charles F. Wagner, Jr. | (1) | $ | — | $ | 577,500 | $ | 1,299,375 | |||||||||||||||||||||||||||
| (2a) | 2/1/2023 | — | 4,720 | 9,440 | $ | 1,500,158 | ||||||||||||||||||||||||||||
| (2b) | 2/1/2023 | — | 4,720 | 9,440 | $ | 750,079 | ||||||||||||||||||||||||||||
| (3) | 2/1/2023 | 9,440 | $ | 3,000,315 | ||||||||||||||||||||||||||||||
| David M. Altshuler | (1) | $ | — | $ | 577,500 | $ | 1,299,375 | |||||||||||||||||||||||||||
| (2a) | 2/1/2023 | — | 3,933 | 7,866 | $ | 1,250,025 | ||||||||||||||||||||||||||||
| (2b) | 2/1/2023 | — | 3,933 | 7,866 | $ | 625,013 | ||||||||||||||||||||||||||||
| (3) | 2/1/2023 | 7,866 | $ | 2,500,051 | ||||||||||||||||||||||||||||||
| Stuart A. Arbuckle | (1) | $ | — | $ | 810,000 | $ | 1,822,500 | |||||||||||||||||||||||||||
| (2a) | 2/1/2023 | — | 5,605 | 11,210 | $ | 1,781,437 | ||||||||||||||||||||||||||||
| (2b) | 2/1/2023 | — | 5,605 | 11,210 | $ | 890,719 | ||||||||||||||||||||||||||||
| (3) | 2/1/2023 | 11,209 | $ | 3,562,556 | ||||||||||||||||||||||||||||||
| Jeffrey M. Leiden | (1) | $ | — | $ | — | $ | — | |||||||||||||||||||||||||||
| (2a) | 2/1/2023 | — | 10,226 | 20,452 | $ | 3,250,130 | ||||||||||||||||||||||||||||
| (2b) | 2/1/2023 | — | — | — | $ | — | ||||||||||||||||||||||||||||
| (3) | 2/1/2023 | 10,226 | $ | 3,250,130 | ||||||||||||||||||||||||||||||
(1)Annual Cash Bonus. The amounts in the “Estimated Possible Payouts Under Non-Equity Incentive Plan Awards” column represent the minimum threshold, target and maximum amounts that our NEOs were eligible to earn pursuant to our 2023 annual cash bonus program. Actual amounts paid to each of the NEOs under this program for 2023 performance are set forth in the Summary Compensation Table above.
(2)PSU. The amounts in the “Estimated Future Payouts Under Equity Incentive Plan Awards” column represent the minimum threshold, target and maximum number of shares that could be earned and vest pursuant to PSUs granted in 2023. Pursuant to U.S. GAAP, the grant date value of the financial PSU awards (2a) was based on 100% of the fair value of the target shares and the grant date value of the non-financial PSU awards (2b) was based on a fair value of 50% of the target shares in 2023. These awards vest if, and only if, performance objectives are achieved, as described in the footnotes to the table Outstanding Equity Awards at Fiscal Year-End for 2023 below.
(3)Time-Based RSUs. The amounts in the “All Other Stock Awards: Number of Shares of Stock or Units” column represent the number of time-based RSUs granted to the NEOs in 2023, which (other than with respect to Dr. Leiden) generally vest annually over three years.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 76
| Name | 401(k) Match | Life Insurance Premiums | Relocation Expense | Total | ||||||||
| Jeffrey M. Leiden | $ | 11,925 | $ | 1,185 | $ | — | $ | 13,110 | ||||
| Ian F. Smith | $ | 11,925 | $ | 1,185 | $ | — | $ | 13,110 | ||||
| David Altshuler | $ | 11,925 | $ | 1,185 | $ | — | $ | 13,110 | ||||
| Stuart A. Arbuckle | $ | 11,925 | $ | 1,185 | $ | — | $ | 13,110 | ||||
| Jeffrey Chodakewitz | $ | 11,925 | $ | 1,185 | $ | 2,144 | $ | 15,254 | ||||
| Back of Contents |
Each NEO has entered into an employment agreement with the company, which provides the executives the right to participate in all of the company’s compensation and benefits plans and equity programs, as described in Compensation Discussion & Analysis.
The following table provides information with respect to outstanding equity awards held by each of our NEOs on December 31, 2023, based on the closing price of $406.89 per share of our common stock on December 29, 2023:
| Option Awards | Stock Awards | ||||||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options Exercisable (shares)(1) | Number of Securities Underlying Unexercised Options Unexercisable (shares) | Option Exercise Price (per share) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (shares) | Market Value of Shares or Units of Stock That Have Not Vested | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (shares) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested | |||||||||||||||||||
| Reshma Kewalramani | Time-based RSU | ||||||||||||||||||||||||||
| 9,677 | (2) | $ | 3,937,475 | ||||||||||||||||||||||||
| 19,412 | (3) | $ | 7,898,549 | ||||||||||||||||||||||||
| 26,972 | (4) | $ | 10,974,637 | ||||||||||||||||||||||||
| Performance-based RSU | |||||||||||||||||||||||||||
| 9,678 | (5) | $ | 3,937,881 | ||||||||||||||||||||||||
| 19,412 | (6) | $ | 7,898,549 | ||||||||||||||||||||||||
| 14,516 | (7) | $ | 5,906,415 | ||||||||||||||||||||||||
| 26,972 | (8) | $ | 10,974,637 | ||||||||||||||||||||||||
| 14,559 | (9) | $ | 5,923,912 | ||||||||||||||||||||||||
| 13,486 | (10) | $ | 5,487,319 | ||||||||||||||||||||||||
| Stock Options | |||||||||||||||||||||||||||
| 1,565 | 0 | $ | 187.53 | 2/5/2029 | |||||||||||||||||||||||
| Charles F. Wagner, Jr. | Time-based RSU | ||||||||||||||||||||||||||
| 3,876 | (2) | $ | 1,577,106 | ||||||||||||||||||||||||
| 6,842 | (3) | $ | 2,783,941 | ||||||||||||||||||||||||
| 9,440 | (4) | $ | 3,841,042 | ||||||||||||||||||||||||
| Performance-based RSU | |||||||||||||||||||||||||||
| 3,876 | (5) | $ | 1,577,106 | ||||||||||||||||||||||||
| 6,842 | (6) | $ | 2,783,941 | ||||||||||||||||||||||||
| 5,814 | (7) | $ | 2,365,658 | ||||||||||||||||||||||||
| 9,440 | (8) | $ | 3,841,042 | ||||||||||||||||||||||||
| 5,131 | (9) | $ | 2,087,753 | ||||||||||||||||||||||||
| 4,720 | (10) | $ | 1,920,521 | ||||||||||||||||||||||||
| Stock Options | |||||||||||||||||||||||||||
| 9,532 | 0 | $ | 189.38 | 4/9/2029 | |||||||||||||||||||||||
| David M. Altshuler | Time-based RSU | ||||||||||||||||||||||||||
| 3,876 | (2) | $ | 1,577,106 | ||||||||||||||||||||||||
| 8,210 | (3) | $ | 3,340,567 | ||||||||||||||||||||||||
| 7,866 | (4) | $ | 3,200,597 | ||||||||||||||||||||||||
| Performance-based RSU | |||||||||||||||||||||||||||
| 3,876 | (5) | $ | 1,577,106 | ||||||||||||||||||||||||
| 8,210 | (6) | $ | 3,340,567 | ||||||||||||||||||||||||
| 5,814 | (7) | $ | 2,365,658 | ||||||||||||||||||||||||
| 7,866 | (8) | $ | 3,200,597 | ||||||||||||||||||||||||
| 6,157 | (9) | $ | 2,505,222 | ||||||||||||||||||||||||
| 3,933 | (10) | $ | 1,600,298 | ||||||||||||||||||||||||
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 77
| Back of Contents |
| Option Awards | Stock Awards | ||||||||||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options Exercisable (shares)(1) | Number of Securities Underlying Unexercised Options Unexercisable (shares) | Option Exercise Price (per share) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (shares) | Market Value of Shares or Units of Stock That Have Not Vested | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (shares) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested | |||||||||||||||||||
| Stuart A. Arbuckle | Time-based RSU | ||||||||||||||||||||||||||
| 4,651 | (2) | $ | 1,892,445 | ||||||||||||||||||||||||
| 9,749 | (3) | $ | 3,966,771 | ||||||||||||||||||||||||
| 11,209 | (4) | $ | 4,560,830 | ||||||||||||||||||||||||
| 12,753 | (11) | $ | 5,189,068 | ||||||||||||||||||||||||
| Performance-based RSU | |||||||||||||||||||||||||||
| 4,652 | (5) | $ | 1,892,852 | ||||||||||||||||||||||||
| 9,750 | (6) | $ | 3,967,178 | ||||||||||||||||||||||||
| 6,976 | (7) | $ | 2,838,465 | ||||||||||||||||||||||||
| 11,210 | (8) | $ | 4,561,237 | ||||||||||||||||||||||||
| 7,312 | (9) | $ | 2,975,180 | ||||||||||||||||||||||||
| 5,605 | (10) | $ | 2,280,618 | ||||||||||||||||||||||||
| 18,811 | (12) | $ | 7,654,008 | ||||||||||||||||||||||||
| Jeffrey M. Leiden | Performance-based RSU | ||||||||||||||||||||||||||
| 10,464 | (7) | $ | 4,257,697 | ||||||||||||||||||||||||
| 20,452 | (8) | $ | 8,321,714 | ||||||||||||||||||||||||
| Stock Options | |||||||||||||||||||||||||||
| 33,524 | 0 | $ | 91.05 | 2/1/2026 | |||||||||||||||||||||||
| 103,550 | 0 | $ | 86.52 | 2/2/2027 | |||||||||||||||||||||||
| (1) | The option expiration dates listed above reflect the final expiration date for each of the listed options. If the NEO’s service with us is terminated, the options would expire, subject to certain exceptions, 90 days after the termination of service. |
| (2) | These time-based RSU awards, which were granted on February 3, 2021, vest in three annual installments. The shares listed on the table above represent the third annual installment, which vested on February 17, 2024. |
| (3) | These time-based RSU awards, which were granted on February 1, 2022, vest in three annual installments. The shares listed on the table above represent the second and third annual installments, which vested on February 24, 2024 and are scheduled to vest February 24, 2025, respectively. |
| (4) | These time-based RSU awards, which were granted on February 1, 2023, vest in three annual installments. The shares listed on the table above represent the three annual installments, the first of which vested on February 10, 2024, and are scheduled to vest in two remaining annual installments on February 10, 2025 and 2026. |
| (5) | This PSU award was based on the achievement of one-year financial performance metrics tied to our net product revenue for medicines for the treatment of CF during 2021. In February 2022, our MDCC certified as to the level of performance at 200% of the number of target shares with the earned shares vesting in annual installments on February 17, 2022, 2023 and 2024. The shares listed on the table above represent the final installment of the earned shares, which vested on February 17, 2024. |
| (6) | This PSU award was based on the achievement of one-year financial performance metrics tied to our net product revenue for medicines for the treatment of CF during 2022, with vesting of the earned shares in three equal installments on each of February 24, 2023, 2024 and 2025. In February 2023, our MDCC certified as to the level of performance at 200% of the number of target shares. The shares listed on the table above represent the second and third installments of earned shares, which vested on February 24, 2024 and are scheduled to vest on February 24, 2025, respectively. |
| (7) | This PSU award is based on the achievement of three-year non-financial performance metrics, which were established by the MDCC on January 20, 2021. The performance conditions associated with the awards consist of multiple clinical and research milestones, with a payout range of zero to 200%. In February 2024, our MDCC certified as to the level of performance at 100% of the number of target shares, with the number of shares reported above reflected as such. The earned shares vested on February 20, 2024. |
| (8) | This PSU award was based on the achievement of one-year financial performance metrics, which were established by the MDCC on February 1, 2023, and are tied to our net product revenue for medicines for the treatment of CF during 2023, with vesting of the earned shares generally occurring in three equal installments scheduled for each of February 10, 2024, 2025 and 2026. In February 2024, our MDCC certified as to the level of performance at 200% of the number of target shares, with the number of shares reported above reflected as such. Dr Leiden’s earned shares fully vested on February 10, 2024 pursuant to the terms of his amended employment agreement. |
| (9) | This PSU award is based on the achievement of three-year non-financial performance metrics, which were established by the MDCC on January 24, 2022, with the number and value of shares reported assuming target performance (100%). The performance conditions associated with the awards consist of multiple clinical and research milestones, with a payout range of zero to 200%. The specific clinical and research milestones are not disclosed for competitive reasons. Performance against these goals will be certified by our MDCC in early 2025. |
| (10) | This PSU award is based on the achievement of three-year non-financial performance metrics, which were established by the MDCC on February 1, 2023, with the number of shares reported assuming target performance (100%). The performance conditions associated with the awards consist of multiple clinical and research milestones, with a payout range of zero to 200%. The specific clinical and research milestones are not disclosed for competitive reasons. Performance against these goals will be certified by our MDCC in early 2026. |
| (11) | This special, one-time time-based RSU award was granted to Mr. Arbuckle in December 2022 and will cliff vest on July 1, 2025. |
| (12) | This special, one-time PSU award was awarded to Mr. Arbuckle in December 2022. The number of shares reported assumes target performance (100%). The performance conditions associated with the awards consist of multiple commercial milestones, with a payout range of zero to 200%. The specific commercial milestones are not disclosed for competitive reasons. Performance against these goals will be certified by our MDCC in July 2025. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 78
The following table sets forth the value realized by our named executive officersNEOs from options to purchase common stock exercised by the named executive officersNEOs during
| Option Awards | Stock Awards | |||||||||||
| Name | Number of Shares Acquired on Exercise | Value Realized on Exercise | Number of Shares Acquired on Vesting | Value Realized on Vesting | ||||||||
| Jeffrey M. Leiden | — | $ | — | 64,500 | $ | 7,739,355 | ||||||
| Ian F. Smith | — | $ | — | 26,742 | $ | 3,290,219 | ||||||
| David Altshuler | 20,000 | $ | 1,229,700 | — | $ | — | ||||||
| Stuart A. Arbuckle | 94,211 | $ | 6,826,163 | 33,117 | $ | 3,771,436 | ||||||
| Jeffrey Chodakewitz | 17,188 | $ | 838,864 | 2,375 | $ | 268,565 | ||||||
| Name | Year | Salary | Annual Cash Bonus | All Other Compensation/Bonus | Value Realized from Vesting of Restricted Stock | Value Realized from Stock Options | Total Realized Compensation | ||||||||||||
| Jeffrey M. Leiden | 2015 | $ | 1,297,692 | $ | 3,463,200 | $ | 13,110 | $ | 7,739,355 | $ | — | $ | 12,513,357 | ||||||
| 2014 | $ | 1,100,000 | $ | 2,970,000 | $ | 12,857 | $ | 5,988,035 | $ | 18,793,100 | $ | 28,863,992 | |||||||
| 2013 | $ | 1,038,462 | $ | 2,772,000 | $ | 12,675 | $ | 4,289,766 | $ | — | $ | 8,112,903 | |||||||
| Ian F. Smith | 2015 | $ | 701,796 | $ | 832,500 | $ | 13,110 | $ | 3,290,219 | $ | — | $ | 4,837,625 | ||||||
| 2014 | $ | 650,000 | $ | 731,250 | $ | 12,857 | $ | — | $ | 7,598,771 | $ | 8,992,878 | |||||||
| 2013 | $ | 582,959 | $ | 682,500 | $ | 12,675 | $ | 393,878 | $ | 34,925,940 | $ | 36,597,952 | |||||||
| David Altshuler | 2015 | $ | 528,846 | $ | 552,628 | $ | 263,110 | $ | — | $ | 1,229,700 | $ | 2,574,284 | ||||||
| Stuart A. Arbuckle | 2015 | $ | 629,262 | $ | 721,500 | $ | 13,110 | $ | 3,771,436 | $ | 6,826,163 | $ | 11,961,471 | ||||||
| 2014 | $ | 600,000 | $ | 675,000 | $ | 12,857 | $ | 1,396,788 | $ | 2,935,124 | $ | 5,619,769 | |||||||
| 2013 | $ | 553,846 | $ | 630,000 | $ | 12,675 | $ | 1,338,439 | $ | — | $ | 2,534,960 | |||||||
| Jeffrey Chodakewitz | 2015 | $ | 615,231 | $ | 617,382 | $ | 15,254 | $ | 268,565 | $ | 838,864 | $ | 2,355,296 | ||||||
| 2014 | $ | 539,077 | $ | 630,000 | $ | 491,936 | $ | — | $ | 248,291 | $ | 1,909,304 | |||||||
| Estimated Possible Payouts Under Non-Equity Incentive Plan Awards | Estimated Future Payouts Under Equity Incentive Plan Awards (shares) | ||||||||||||||||||||||||||||||||
Name | Grant Date | Threshold ($) | Target ($) | Maximum ($) | Threshold (#) | Target (#) | Maximum (#) | All Other Stock Awards: Number of Shares of Stock or Units (#) | All Other Option Awards: Number of Securities Underlying Options (#) | Exercise or Base Price of Option Awards ($/Sh) | Closing Price of Stock on Grant Date ($/Sh) | Grant-Date Fair Value of Stock and Option Awards ($) | |||||||||||||||||||||
| Jeffrey M. | $ | 0 | $ | 1,560,000 | $ | 3,510,000 | |||||||||||||||||||||||||||
| Leiden | 2/3/2015 | 64,500 | $ | 7,038,885 | |||||||||||||||||||||||||||||
| 2/3/2015 | 213,000 | $ | 109.14 | $ | 108.72 | $ | 10,133,326 | ||||||||||||||||||||||||||
| 7/21/2015 | 106,500 | $ | 131.89 | $ | 130.97 | $ | 6,153,613 | ||||||||||||||||||||||||||
| Ian F. | $ | 0 | $ | 375,000 | $ | 843,750 | |||||||||||||||||||||||||||
| Smith | 2/3/2015 | 20,700 | $ | 2,258,991 | |||||||||||||||||||||||||||||
| 2/3/2015 | 68,000 | $ | 109.14 | $ | 108.72 | $ | 3,235,052 | ||||||||||||||||||||||||||
| 7/21/2015 | 34,000 | $ | 131.89 | $ | 130.97 | $ | 1,964,534 | ||||||||||||||||||||||||||
| David | $ | 0 | $ | 275,000 | $ | 618,750 | |||||||||||||||||||||||||||
| Altshuler | 1/12/2015 | — | 75,000 | 75,000 | $ | 9,078,750 | |||||||||||||||||||||||||||
| 7/21/2015 | 34,000 | 131.89 | 130.97 | $ | 1,964,534 | ||||||||||||||||||||||||||||
| Stuart A. | $ | 0 | $ | 325,000 | $ | 731,250 | |||||||||||||||||||||||||||
| Arbuckle | 2/3/2015 | 20,700 | $ | 2,258,991 | |||||||||||||||||||||||||||||
| 2/3/2015 | 68,000 | $ | 109.14 | $ | 108.72 | $ | 3,235,052 | ||||||||||||||||||||||||||
| 7/21/2015 | 34,000 | $ | 131.89 | $ | 130.97 | $ | 1,964,534 | ||||||||||||||||||||||||||
| Jeffrey | $ | 0 | $ | 309,000 | $ | 695,250 | |||||||||||||||||||||||||||
| Chodakewitz | 2/3/2015 | 17,250 | $ | 1,882,493 | |||||||||||||||||||||||||||||
| 2/3/2015 | 57,500 | $ | 109.14 | $ | 108.72 | $ | 2,735,522 | ||||||||||||||||||||||||||
| 7/21/2015 | 34,000 | $ | 131.89 | $ | 130.97 | $ | 1,964,534 | ||||||||||||||||||||||||||
| Option Awards | Stock Awards | |||||||||||||||
| Name | Number of Shares Acquired on Exercise | Value Realized on Exercise | Number of Shares Acquired on Vesting | Value Realized on Vesting | ||||||||||||
| Reshma Kewalramani | 17,135 | $ | 3,858,352 | 59,413 | $ | 17,405,936 | ||||||||||
| Charles F. Wagner, Jr. | — | $ | — | 31,799 | $ | 9,352,864 | ||||||||||
| David M. Altshuler | 1,304 | $ | 154,486 | 36,607 | $ | 10,768,987 | ||||||||||
| Stuart A. Arbuckle | 17,206 | $ | 3,208,405 | 39,696 | $ | 11,667,856 | ||||||||||
| Jeffrey M. Leiden | — | $ | — | 76,578 | $ | 22,721,383 | ||||||||||
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 70
| Back of Contents |
| Option Awards | Stock Awards | ||||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options Exercisable (shares) (1) | Number of Securities Underlying Unexercised Options Unexercisable (shares) (1) | Option Exercise Price (per share) | Option Expiration Date (2) | Number of Shares or Units of Stock That Have Not Vested (shares) | Market Value of Shares or Units of Stock That Have Not Vested | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (shares) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested | |||||||||||
| Jeffrey M. Leiden | Restricted Stock | ||||||||||||||||||
| 19,667 | (3) | $ | 2,474,699 | ||||||||||||||||
| 64,500 | (4) | $ | 8,116,035 | ||||||||||||||||
| 125,000 | (5) | $ | 15,728,750 | ||||||||||||||||
| Stock Options | |||||||||||||||||||
| 213,108 | 0 | $29.98 | 12/13/2021 | ||||||||||||||||
| 30,000 | 0 | $34.05 | 7/5/2019 | ||||||||||||||||
| 20,000 | 0 | $34.24 | 5/31/2020 | ||||||||||||||||
| 1,527 | 0 | $34.39 | 12/14/2020 | ||||||||||||||||
| 121,687 | 55,313 | $45.11 | 2/4/2023 | ||||||||||||||||
| 95,875 | 22,125 | $48.74 | 7/24/2022 | ||||||||||||||||
| 22,500 | 0 | $53.85 | 5/31/2021 | ||||||||||||||||
| 93,187 | 119,813 | $77.31 | 2/4/2024 | ||||||||||||||||
| 59,906 | 46,594 | $83.36 | 7/29/2023 | ||||||||||||||||
| 33,281 | 73,219 | $96.87 | 7/14/2024 | ||||||||||||||||
| 39,937 | 173,063 | $109.14 | 2/2/2025 | ||||||||||||||||
| 6,656 | 99,844 | $131.89 | 7/20/2025 | ||||||||||||||||
| Ian F. Smith | Restricted Stock | ||||||||||||||||||
| 6,042 | (6) | $ | 760,265 | ||||||||||||||||
| 6,042 | (3) | $ | 760,265 | ||||||||||||||||
| 20,700 | (4) | $ | 2,604,681 | ||||||||||||||||
| 75,000 | (5) | $ | 9,437,250 | ||||||||||||||||
| Stock Options | |||||||||||||||||||
| 20,390 | 3,399 | $37.86 | 2/1/2022 | ||||||||||||||||
| 10,195 | 0 | $38.80 | 2/2/2021 | ||||||||||||||||
| 20,391 | 16,992 | $45.11 | 2/4/2023 | ||||||||||||||||
| 11,327 | 6,797 | $48.74 | 7/24/2022 | ||||||||||||||||
| 9,062 | 0 | $51.75 | 7/12/2021 | ||||||||||||||||
| 29,750 | 38,250 | $77.31 | 2/4/2024 | ||||||||||||||||
| 19,125 | 14,875 | $83.36 | 7/29/2023 | ||||||||||||||||
| 10,625 | 23,375 | $96.87 | 7/14/2024 | ||||||||||||||||
| 12,750 | 55,250 | $109.14 | 2/2/2025 | ||||||||||||||||
| 2,125 | 31,875 | $131.89 | 7/20/2025 | ||||||||||||||||
| Option Awards | Stock Awards | |||||||||||||||||
| Name | Number of Securities Underlying Unexercised Options Exercisable (shares) (1) | Number of Securities Underlying Unexercised Options Unexercisable (shares) (1) | Option Exercise Price (per share) | Option Expiration Date (2) | Number of Shares or Units of Stock That Have Not Vested (shares) | Market Value of Shares or Units of Stock That Have Not Vested | Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (shares) | Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested | ||||||||||
| David Altshuler | Restricted Stock | |||||||||||||||||
| 75,000 | (5) | $ | 9,437,250 | |||||||||||||||
| Stock Options | ||||||||||||||||||
| 3,750 | 3,750 | $63.14 | 5/23/2022 | |||||||||||||||
| 7,500 | 0 | $72.14 | 5/31/2024 | |||||||||||||||
| 20,000 | 0 | $81.54 | 5/31/2023 | |||||||||||||||
| 2,125 | 31,875 | $131.89 | 7/20/2025 | |||||||||||||||
| Stuart A. Arbuckle | Restricted Stock | |||||||||||||||||
| 2,416 | (7) | $ | 304,005 | |||||||||||||||
| 6,042 | (3) | $ | 760,265 | |||||||||||||||
| 20,700 | (4) | $ | 2,604,681 | |||||||||||||||
| 75,000 | (5) | $ | 9,437,250 | |||||||||||||||
| Stock Options | ||||||||||||||||||
| 0 | 28,320 | $45.11 | 2/4/2023 | |||||||||||||||
| 0 | 13,594 | $53.74 | 9/3/2022 | |||||||||||||||
| 0 | 38,250 | $77.31 | 2/4/2024 | |||||||||||||||
| 19,125 | 14,875 | $83.36 | 7/29/2023 | |||||||||||||||
| 10,625 | 23,375 | $96.87 | 7/14/2024 | |||||||||||||||
| 12,750 | 55,250 | $109.14 | 2/2/2025 | |||||||||||||||
| 2,125 | 31,875 | $131.89 | 7/20/2025 | |||||||||||||||
| Jeffrey Chodakewitz | Restricted Stock | |||||||||||||||||
| 7,125 | (8) | $ | 896,539 | |||||||||||||||
| 17,250 | (4) | $ | 2,170,568 | |||||||||||||||
| 75,000 | (5) | $ | 9,437,250 | |||||||||||||||
| Stock Options | ||||||||||||||||||
| 3,437 | 30,938 | $73.51 | 1/1/2024 | |||||||||||||||
| 5,156 | 18,907 | $96.87 | 7/14/2024 | |||||||||||||||
| 10,781 | 46,719 | $109.14 | 2/2/2025 | |||||||||||||||
| 2,125 | 31,875 | $131.89 | 7/20/2025 | |||||||||||||||
The amounts shown in the following table are calculated based on the amounts that would have been payable by us had the listed named executive officercurrent NEO experienced an employment termination on
| Voluntary Termination or Retirement/Termination for Cause | Separate From a Change of Control Involuntary Termination Other Than for Cause/ Termination by Executive With Good Reason | In Connection With a Change of Control Involuntary Termination Other Than for Cause/ Termination by Executive for Good Reason | Disability | Death | |||||||||||
| Jeffrey M. Leiden | |||||||||||||||
| Cash Severance Benefits | $ | — | $ | 7,280,000 | $ | 10,111,400 | $ | 1,560,000 | $ | 1,560,000 | |||||
| Continuation of Employee Benefits | — | 27,553 | 35,504 | — | — | ||||||||||
| Accelerated Vesting of Stock Options | — | — | 18,971,499 | 10,651,733 | 18,971,499 | ||||||||||
| Continued Vesting of Stock Options | — | 12,898,752 | — | — | — | ||||||||||
| Accelerated Vesting of Restricted Stock | — | 2,474,699 | 26,319,484 | 6,961,748 | 26,319,484 | ||||||||||
| Total | $ | — | $ | 22,681,004 | $ | 55,437,887 | $ | 19,173,481 | $ | 46,850,983 | |||||
| Ian F. Smith | |||||||||||||||
| Cash Severance Benefits | $ | — | $ | 1,500,000 | $ | 1,500,000 | $ | 937,500 | $ | 937,500 | |||||
| Continuation of Employee Benefits | — | 18,369 | 18,369 | — | — | ||||||||||
| Accelerated Vesting of Stock Options | — | 4,768,173 | 6,281,279 | 3,636,003 | 6,281,279 | ||||||||||
| Accelerated Vesting of Restricted Stock | — | 8,123,554 | 13,562,461 | 7,020,374 | 13,562,461 | ||||||||||
| 280G Excise Tax | — | — | — | — | — | ||||||||||
| Total | $ | — | $ | 14,410,096 | $ | 21,362,109 | $ | 11,593,877 | $ | 20,781,240 | |||||
| David Altshuler | |||||||||||||||
| Cash Severance Benefits | $ | — | $ | 825,000 | $ | 1,100,000 | $ | — | $ | — | |||||
| Continuation of Employee Benefits | — | 23,669 | 23,669 | — | — | ||||||||||
| Accelerated Vesting of Stock Options | — | — | 235,088 | — | 235,088 | ||||||||||
| Accelerated Vesting of Restricted Stock | — | — | 9,437,250 | — | 9,437,250 | ||||||||||
| Total | — | 848,669 | 10,796,007 | — | 9,672,338 | ||||||||||
| Stuart A. Arbuckle | |||||||||||||||
| Cash Severance Benefits | $ | — | $ | 975,000 | $ | 1,300,000 | $ | — | $ | — | |||||
| Continuation of Employee Benefits | — | 23,669 | 23,669 | — | — | ||||||||||
| Accelerated Vesting of Stock Options | — | — | 7,352,676 | — | 7,352,676 | ||||||||||
| Accelerated Vesting of Restricted Stock | — | — | 13,106,201 | — | 13,106,201 | ||||||||||
| Total | $ | — | $ | 998,669 | $ | 21,782,546 | $ | — | $ | 20,458,877 | |||||
| Jeffrey Chodakewitz | |||||||||||||||
| Cash Severance Benefits | $ | — | $ | 927,000 | $ | 1,236,000 | $ | — | $ | — | |||||
| Continuation of Employee Benefits | — | 23,420 | 23,420 | — | — | ||||||||||
| Accelerated Vesting of Stock Options | — | — | 2,945,963 | — | 2,945,963 | ||||||||||
| Accelerated Vesting of Restricted Stock | — | 896,539 | 12,504,356 | — | 12,504,356 | ||||||||||
| Total | $ | — | $ | 1,846,959 | $ | 16,709,739 | $ | — | $ | 15,450,319 | |||||
| Voluntary Termination or Retirement/ Termination for Cause | Separate From a Change of Control, Involuntary Termination Other Than for Cause/ Termination by Executive for Good Reason | In Connection With a Change of Control, Involuntary Termination Other Than for Cause/ Termination by Executive for Good Reason | Disability | Death | |||||||||||||||
| Reshma Kewalramani | |||||||||||||||||||
| Cash Severance Benefits | $ | — | $ | 8,400,000 | $ | 11,667,000 | $ | 1,800,000 | $ | 1,800,000 | |||||||||
| Continuation of Employee Benefits | — | 44,559 | 44,559 | — | — | ||||||||||||||
| Accelerated Vesting of Restricted Stock Units | — | 27,167,232 | 57,452,055 | 57,452,055 | 57,452,055 | ||||||||||||||
| TOTAL | $ | — | $ | 35,611,791 | $ | 69,163,614 | $ | 59,252,055 | $ | 59,252,055 | |||||||||
| Charles F. Wagner, Jr. | |||||||||||||||||||
| Cash Severance Benefits | $ | — | $ | 1,402,500 | $ | 1,980,000 | $ | — | $ | — | |||||||||
| Continuation of Employee Benefits | — | 26,638 | 26,638 | — | — | ||||||||||||||
| Accelerated Vesting of Restricted Stock Units | — | — | 20,857,589 | 20,857,589 | 20,857,589 | ||||||||||||||
| TOTAL | $ | — | $ | 1,429,138 | $ | 22,864,227 | $ | 20,857,589 | $ | 20,857,589 | |||||||||
| David M. Altshuler | |||||||||||||||||||
| Cash Severance Benefits | $ | — | $ | 1,402,500 | $ | 1,980,000 | $ | — | $ | — | |||||||||
| Continuation of Employee Benefits | — | 29,706 | 29,706 | — | — | ||||||||||||||
| Accelerated Vesting of Restricted Stock Units | — | — | 21,107,419 | 21,107,419 | 21,107,419 | ||||||||||||||
| TOTAL | $ | — | $ | 1,432,206 | $ | 23,117,125 | $ | 21,107,419 | $ | 21,107,419 | |||||||||
| Stuart A. Arbuckle | |||||||||||||||||||
| Cash Severance Benefits | $ | — | $ | 1,710,000 | $ | 2,520,000 | $ | — | $ | — | |||||||||
| Continuation of Employee Benefits | — | 29,706 | 29,706 | — | — | ||||||||||||||
| Accelerated Vesting of Restricted Stock Units | — | — | 39,498,033 | 39,498,033 | 39,498,033 | ||||||||||||||
| TOTAL | $ | — | $ | 1,739,706 | $ | 42,047,739 | $ | 39,498,033 | $ | 39,498,033 | |||||||||
| Jeffrey M. Leiden | |||||||||||||||||||
| Cash Severance Benefits | $ | — | $ | 13,000,000 | $ | 13,000,000 | $ | 6,500,000 | $ | 6,500,000 | |||||||||
| Continuation of Employee Benefits | — | 29,397 | 29,397 | — | — | ||||||||||||||
| Accelerated Vesting of Restricted Stock Units | — | — | — | — | — | ||||||||||||||
| TOTAL | $ | — | $ | 13,029,397 | $ | 13,029,397 | $ | 6,500,000 | $ | 6,500,000 | |||||||||
The amounts in the table above do not include any life insurance payments or disability insurance payments that the executive or the executive’s estate may receive under existing insurance policies. The assumptions underlying the calculations in the table include:
 | No amounts have been included with respect to stock options held by NEOs as all of their outstanding stock options are fully vested. |
 | The value of each share of restricted stock unit that would be accelerated or continue to vest, in each case in the circumstances described below, equals $406.89 per share (the closing price on the last trading day of 2023). The value of any PSUs that have not been certified as to the level of performance by the MDCC as of December 31, 2023 are reported above assuming target performance (100%). |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 80
| Back of Contents |
 | Our board of directors would elect not to pay a pro rata portion of an executive’s target bonus for the year of termination in cases where the executive’s employment is terminated voluntarily by the executive (for any reason, including retirement) or for cause, under our policy that cash bonuses are payable only to employees who are otherwise eligible and who remain employed by us on the date of bonus payment, typically in February of the next year. |
 | Our board of directors would have assigned the same 2023 individual and company performance ratings on December 31, 2023 as they assigned in the first quarter of 2024. |
 | PSUs granted to Dr. Leiden in his current role as Executive Chairman are not subject to service-based vesting conditions and will remain eligible to vest following certification of the corresponding performance criteria. |
 | No NEO who has met the age and service requirements for retirement has provided the required notice for a termination of employment to qualify as a retirement as of December 31, 2023. |
The actual amounts that the named executive officerscurrent NEOs could receive in the future as a result of a termination of employment would likely differ materially from the amounts set forth above as a result of, among other things, changes in our stock price, changes in theirthe officers’ base salary, target bonus amounts and actual bonus amounts, and the vesting and grants of additional equity awards.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 75
| Back of Contents |
We have entered into agreements and maintain plans that will require us to provide to our named executive officersNEOs cash compensation, benefits, and/or acceleration of the vesting of equity awards in the event of termination of employment or service as a director under specified circumstances. The following summary descriptions of such agreements with our named executive officers are qualified by the complete terms and conditions set forth in each of the agreements. In addition to the agreements described below, outstanding options granted under our stock and option plans provide that, in the event of certain changes of control, either appropriate provision for the continuation of all then-outstanding options must be made, or the vesting of those options will be accelerated and they will become fully exercisable immediately prior to such change of control. All options held by our NEOs are fully vested and, as a result, would not have their vesting accelerated as a result of change in control or otherwise. As described below, the benefits that are to be provided in connection with a change of control are subject to a “double trigger.” A change of control does not itself trigger benefits; rather, benefits are paid only if the employment of the executive is terminated by us other than for cause, death or disability or by the executive for good reason during a specified period before or after a change of control.
In addition to the benefits described below, under programs applicable to all employees, if a named executive officeran NEO dies while an employee, histheir estate and/or beneficiaries would receive full acceleration of all outstanding stock optionsequity awards, and restricted stock awards.if an NEO’s employment is terminated due to disability, they would receive full acceleration of equity grants made. None of our current employment agreements provide for a so-called Section 4999 excise tax “gross-up,” and we have a policy against providing so-called Section 4999 excise tax “gross-up” in the future.
Dr. Kewalramani’s written employment agreement provides that she is entitled to receive compensation as determined by our board of directors and is eligible to receive the benefits generally made available to our executives. In addition, Dr. Kewalramani has agreed not to engage in specified competitive activities for 12 months after her employment with us terminates.
If (i) Dr. Kewalramani’s employment is terminated by us without cause or (ii) she terminates her employment for good reason, she would be entitled to receive, subject to limited exceptions:
| Severance Payment: | A) 200% of the sum of her (i) base salary at the time of termination and (ii) target bonus for the year in which her employment is terminated B) Any annual bonus for the year prior to the year in which the termination occurs, if not yet paid C) A pro-rated bonus for the year in which the termination occurs based on her target bonus for the year in which the termination occurs |
| Equity: | Outstanding options and RSUs unvested on the termination date would receive partial vesting based on the portion of the award(s) that would have vested during the 12-month period following the termination date. |
| Employee Benefits: | Continuation of certain employee benefits for up to 18 months |
If (i) Dr. Kewalramani’s employment is terminated by us without cause or (ii) she terminates her employment for good reason, in each case, within 90 days prior to or 12 months after a change of control of the company, she would instead be entitled to receive:
| Severance Payment: | A) 299% of the sum of her (i) base salary at the time of termination and (ii) target bonus for the year in which her employment is terminated B) A pro-rated bonus for the year in which the termination occurs C) All cash incentive awards earned by Dr. Kewalramani, if not yet paid |
| Equity: | Full vesting of all outstanding options and restricted stock unit awards (using target or earned shares, as applicable, for performance-based awards) |
| Employee Benefits: | Continuation of certain employee benefits for up to 18 months |
Severance payments to Dr. Kewalramani in connection with a change of control may be reduced to increase their value to Dr. Kewalramani if such payments would be subject to an excise tax under Section 4999 of the Code.
If Dr. Kewalramani’s employment is terminated as a result of death or disability, she would be entitled to receive:
 | a pro-rated bonus for the year of employment termination; |
 | for equity awards not covered by the company-wide equity program described above, vesting of any options then unvested at the time of termination. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 82
| Back of Contents |
Employment Agreements
The terms and conditions of Mr. Wagner’s, Dr. Leiden’sAltshuler’s, and Mr. Arbuckle’s employment are governed by a written employment contract, which wascontracts that were entered into on December 14, 2011, as amended on December 10, 2014, and expires on December 31, 2017, provided that, if Dr. Leiden’sat the time the respective officers joined our company. Each of these officer’s employment with us extends beyond the contract expiration date, certain provisions providing for severance benefits upon a “not-for-cause” employment termination by the company survive the contract expiration. Dr. Leiden’s employment agreementagreements provides that he is entitled to receive compensation as determined by our board of directors and is eligible to receive the benefits generally made available to our executives. In addition, Dr. Leiden has agreed not to engage in specified competitive activities for 18 months after his employment with us terminates.
Under each | ||
| Severance Payment: | The sum of his (i) base salary at the time of termination and (ii) target bonus for the year in which his employment is terminated |
| Employee Benefits: | Continuation of certain employee benefits for up to 12 months |
Change of Control Agreement
We have a change of control agreement with each of Dr. Altshuler, whichMr. Arbuckle, and Mr. Wagner that was entered into in December 2014.at the time the respective officer joined our company. Under this agreement and the executive’s equity agreements, if we terminate Dr. Altshuler’sthe employment of the officer without cause on a date within the 90 days prior to or the 12 months after a change of control or heany of these individuals terminates his employment within 30 days of an event giving rise to a right to terminate for good reason, subject to notice and cure provisions, and the event occurs on a date within the 90 days prior to or the 12 months after a change of control, he would be entitled to receive:
| Severance Payment: | A) | The sum of his (i) base salary at the time of termination and (ii) target bonus for the year in which his employment is terminated |
B) | A pro rata portion of his target bonus for the year in which the termination occurs | |
| Full vesting of all outstanding options | ||
| Employee Benefits: | Continuation of certain employee benefits for up to 12 months | |
Severance payments to Dr. Altshulerthe officer in connection with a change of control may be reduced to increase their value to Dr. Altshulerthe applicable officer if such payments would be subject to an excise tax under Section 4999 of the Code.
Dr. Altshuler’s agreements do not provide for a so-called Section 4999 excise tax “gross-up.”
If (a) Dr. Leiden’s employment is terminated by us without cause or (ii)(b) he terminates his employment with us for good reason, within 30 days of the event giving rise to his right to terminate for good reason, subject to notice and cure provisions, he would be entitled to receive:
If Dr. Leiden’s employment for good reason upon the occurrenceis terminated as a result of the following events without his consent:
Consistent with a program applicable to all our employees, in March 2020, when he completed his service to us as CEO and President, Dr. Leiden received acceleration of control agreement;
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 82
Under the Dodd-Frank Wall Street Reform and Consumer Protection Act, the SEC requires annual disclosure of the ratio of the annual total compensation of our CEO to that of our median employee.
In 2023, Dr. Kewalramani’s annual total compensation, as reported in the Summary Compensation Table, was $20,594,441 and the median of the annual total compensation of all employees of the company (other than our Chief Executive Officer) was $247,549. The ratio of annual total compensation for Dr. Kewalramani to that of our median employee’s annual total compensation was approximately 83:1.
Due to growth in our employee population, we identified a new median employee using target total annual compensation in 2023. Our measure of compensation for identifying the median employee was consistently applied to all employees (converting all non-USD currencies into USD based on 12-month foreign exchange rates for the 12-month period ending October 1, 2023) and includes:
 | Base salary |
 | Target cash bonus |
 | |
The methodology included all 5,294 company employees across 21 countries as of the following events without his consent:
This pay ratio is a member of our executive management team;
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 84
Pursuant to Item 402(v) of Regulation S-K, we are presenting information that describes the relationship between compensation actually paid, as computed under the rules prescribed by Item 402(v), to our NEOs and certain financial performance measures for the company’s five most recently completed fiscal years. For more information about our executive compensation program, refer to the Compensation Discussion and Analysis section of this proxy statement starting on page 50.
| Average | |||||||||||||||||||||||||||||||||||||||
| Summary | Average | Total Shareholder | |||||||||||||||||||||||||||||||||||||
| Summary | Summary | Compensation | Compensation | Return (Value of Initial | |||||||||||||||||||||||||||||||||||
| Compensation | Compensation | Compensation | Compensation | Table Total | Actually Paid | Fixed $100 Investment) | |||||||||||||||||||||||||||||||||
| Table Total for | Table Total for | Actually Paid to | Actually Paid to | for Non-CEO | to Non-CEO | VRTX | Net Product | ||||||||||||||||||||||||||||||||
| Year | CEO(1) | CEO(2) | CEO(1)(3) | CEO(2)(4) | NEOs(5) | NEOs(6) | TSR(7) | NBI TSR(8) | Net Income(9) | Revenue(10) | |||||||||||||||||||||||||||||
| 2023 | $ | 20,594,441 | N/A | $ | 44,202,174 | N/A | $ | 7,376,771 | $ | 18,104,165 | $ | 245.54 | $ | 148.72 | $ | 3.6 Billion | $ | 9.9 Billion | |||||||||||||||||||||
| 2022 | $ | 15,864,497 | N/A | $ | 29,529,089 | N/A | $ | 8,982,257 | $ | 18,263,330 | $ | 174.27 | $ | 142.19 | $ | 3.3 Billion | $ | 8.9 Billion | |||||||||||||||||||||
| 2021 | $ | 15,198,856 | N/A | $ | 18,341,489 | N/A | $ | 7,193,835 | $ | 8,969,051 | $ | 132.52 | $ | 158.20 | $ | 2.3 Billion | $ | 7.6 Billion | |||||||||||||||||||||
| 2020 | $ | 9,111,359 | $ | 16,473,245 | $ | 12,172,454 | $ | 31,319,824 | $ | 6,697,408 | $ | 10,584,384 | $ | 142.62 | $ | 158.17 | $ | 2.7 Billion | $ | 6.2 Billion | |||||||||||||||||||
| 2019 | N/A | $ | 18,789,985 | N/A | $ | 37,124,321 | $ | 4,668,405 | $ | 7,828,475 | $ | 132.13 | $ | 125.11 | $ | 1.2 Billion | $ | 4.2 Billion | |||||||||||||||||||||
| (1) | This column reflects the amounts reported in the “Total” column of the Summary Compensation Table for Reshma Kewalramani, our CEO for a portion of 2020 (effective April 1, 2020), and for 2021, 2022 and 2023. |
| (2) | This column reflects the amounts reported in the “Total” column of the Summary Compensation Table for Jeffrey Leiden, our CEO for 2019 and a portion of 2020 (until April 1, 2020). |
| (3) | The amounts in this column reflect the Compensation Actually Paid for Reshma Kewalramani, our CEO for a portion of 2020 (effective April 1, 2020), and for 2021, 2022 and 2023. The amounts in the following table reflect the adjustments (additions/deductions) to the Summary Compensation Table Total to determine Compensation Actually Paid. Adjustments (additions/deductions) are not listed for Pension/Non-Qualified Deferred Compensation because we do not offer these plans. |
| (4) | The amounts in this column reflect the Compensation Actually Paid for Jeffrey Leiden, our CEO for 2019 and a portion of 2020 (until April 1, 2020). The amounts in the following table reflect the adjustments (additions/deductions) to the Summary Compensation Table Total to determine Compensation Actually Paid. Adjustments (additions/deductions) are not listed for Pension/Non-Qualified Deferred Compensation because we do not offer these plans. |
| (5) | This column reflects the average of the amounts reported in the “Total” column of the Summary Compensation Table for our NEOs as a group (excluding our CEO or CEOs, in the case of 2020) for each of the periods presented. The NEOs included for purposes of computing the amounts in this column were as follows: for 2023 and 2022, Mr. Wagner, Dr. Altshuler, Mr. Arbuckle, and Dr. Leiden; for 2021, Mr. Wagner, Mr. Arbuckle, Dr. Leiden, and Nia Tatsis; for 2020, Mr. Wagner, Dr. Altshuler, Mr. Arbuckle, and Michael Parini; and for 2019, Ms. Kewalramani, Mr. Wagner, Mr. Arbuckle, Amit Sachdev, Paul Silva and Ian Smith. |
| (6) | This column reflects the average of the Compensation Actually Paid for our NEOs as a group (excluding our CEO or CEOs, in the case of 2020) for each of the periods presented. The amounts in the following table reflect the adjustments to the Summary Compensation Table Total to determine Compensation Actually Paid. The NEOs included for purposes of computing the amounts in this column are listed in footnote (5) above. Adjustments (additions/deductions) are not listed for Pension/Non-Qualified Deferred Compensation because we do not offer these plans. |
| (7) | This column represents our cumulative total shareholder return (“TSR”) under SEC rules from December 31, 2018, the last trading day before the start of 2019, through the last trading day for the applicable fiscal year in the table. TSR is calculated by dividing the sum of the cumulative amount of dividends for the measurement period, assuming dividend reinvestment, and the difference between our share price at the end and the beginning of the measurement period by our share price at the beginning of the measurement period. |
| (8) | This column represents the TSR of the NBI, our selected peer group, which is the same peer group used for purposes of Item 201(e) of Regulation S-K from December 31, 2018, the last trading day before the start of 2019, through the last trading day for the applicable fiscal year in the table, assuming reinvestment of dividends and weighted according to the respective companies’ stock market capitalization at the beginning of each period for which a return is indicated. |
| (9) | This column reflects net income as reported for each year in our Annual Report on Form 10-K. |
| (10) | This column reflects net product revenue as reported for each year in our Annual Report on Form 10-K. For purposes of the table above, we have selected net product revenue as the financial performance measure representing the most important financial performance measure used to link executive compensation actually paid to our financial performance in the most recently completed fiscal year. Please the Compensation Discussion and Analysis section beginning on page 50 of this proxy statement for additional information. |
| (1) | This column reflects the amounts reported in the “Total” column of the Summary Compensation Table for Reshma Kewalramani, our CEO for a portion of 2020 (effective April 1, 2020), and for 2021, 2022 and 2023. |
| (2) | This column reflects the amounts reported in the “Total” column of the Summary Compensation Table for Jeffrey Leiden, our CEO for 2019 and a portion of 2020 (until April 1, 2020). |
| (3) | The amounts in this column reflect the Compensation Actually Paid for Reshma Kewalramani, our CEO for a portion of 2020 (effective April 1, 2020), and for 2021, 2022 and 2023. The amounts in the following table reflect the adjustments (additions/deductions) to the Summary Compensation Table Total to determine Compensation Actually Paid. Adjustments (additions/deductions) are not listed for Pension/Non-Qualified Deferred Compensation because we do not offer these plans. |
| Year | Summary Compensation Table Total | Amounts Deducted from Grant Date Value of Annual Equity | Amounts Added for the Fair Value of Awards Granted During Fiscal Year | Amounts Added for Awards Granted & Vested During Fiscal Year | Amounts Add/ Deducted for Awards that Vested During Fiscal Year | Amounts Added/ Deducted for the Change in Fair Value of Awards Outstanding at Fiscal Year End(a) | Compensation Actually Paid | |||||||||||||||
| 2023 | $ | 20,594,441 | $ | (15,001,894) | $ | 24,692,933 | $ | — | $ | 272,767 | $ | 13,643,927 | $ | 44,202,174 | ||||||||
| 2022 | $ | 15,864,497 | $ | (10,640,784) | $ | 16,817,103 | $ | — | $ | 768,712 | $ | 6,719,561 | $ | 29,529,089 | ||||||||
| 2021 | $ | 15,198,856 | $ | (10,924,599) | $ | 14,344,492 | $ | — | $ | (659,323) | $ | 382,063 | $ | 18,341,489 | ||||||||
| 2020 | $ | 9,111,359 | $ | (5,250,411) | $ | 6,587,268 | $ | — | $ | 345,685 | $ | 1,378,553 | $ | 12,172,454 | ||||||||
| (a) | Pursuant to applicable SEC rules, the fair values at the end of each fiscal year for the financial PSU awards were valued at 200% as of December 31, 2023, 2022, 2021, 2020 and 2019; and, the non-financial PSU award for 2020 was valued at 200%, 50% and 50% as of December 31, 2022, 2021 and 2020, respectively; the non-financial PSU award for 2021 was valued at 100%, 50% and 50% as of December 31, 2023, 2022 and 2021, respectively; the non-financial PSU award for 2022 was valued at 50% and 0% as of December 31, 2023 and 2022, respectively; the non-financial PSU award for 2023 was valued at 50% as of December 31, 2023. |
| (4) | The amounts in this column reflect the Compensation Actually Paid for Jeffrey Leiden, our CEO for 2019 and a portion of 2020 (until April 1, 2020). The amounts in the following table reflect the adjustments (additions/deductions) to the Summary Compensation Table Total to determine Compensation Actually Paid. Adjustments (additions/deductions) are not listed for Pension/Non-Qualified Deferred Compensation because we do not offer these plans. |
| Year | Summary Compensation Table Total | Amounts Deducted from Grant Date Value of Annual Equity | Amounts Added for the Fair Value of Awards Granted During Fiscal Year | Amounts Added for Awards Granted & Vested During Fiscal Year | Amounts Add/ Deducted for Awards that Vested During Fiscal Year | Amounts Added/ Deducted for the Change in Fair Value of Awards Outstanding at Fiscal Year End(a) | Compensation Actually Paid | ||||||||||||||
| 2020 | $ | 16,473,245 | $ | (13,335,168) | $ | 9,294,661 | $ | 7,362,423 | $ | 5,724,395 | $ | 5,800,268 | $ | 31,319,824 | |||||||
| 2019 | $ | 18,789,985 | $ | (13,906,720) | $ | 17,220,100 | $ | 509,338 | $ | 4,201,319 | $ | 10,310,299 | $ | 37,124,321 | |||||||
| (a) | Pursuant to applicable SEC rules, the fair values at the end of each fiscal year for the financial PSU awards were valued at 200% as of December 31, 2020, 2019 and 2018; and, the non-financial PSU award for 2016 was valued at 200% as of December 31, 2018; the non-financial PSU award for 2017 was valued at 200% as of December 31, 2019 and 2018; the non-financial PSU award for 2018 was valued at 200%, 100% and 100% as of December 31, 2020, 2019 and 2018, respectively; the non-financial PSU award for 2019 was valued at 100% and 50% as of December 31, 2020 and 2019, respectively; the non-financial PSU award for 2020 was valued at 50% as of December 31, 2020. |
| (5) | This column reflects the average of the amounts reported in the “Total” column of the Summary Compensation Table for our NEOs as a group (excluding our CEO or CEOs, in the case of 2020) for each of the periods presented. The NEOs included for purposes of computing the amounts in this column were as follows: for 2023 and 2022, Mr. Wagner, Dr. Altshuler, Mr. Arbuckle, and Dr. Leiden; for 2021, Mr. Wagner, Mr. Arbuckle, Dr. Leiden, and Nia Tatsis; for 2020, Mr. Wagner, Dr. Altshuler, Mr. Arbuckle, and Michael Parini; and for 2019, Ms. Kewalramani, Mr. Wagner, Mr. Arbuckle, Amit Sachdev, Paul Silva and Ian Smith. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 85
| (6) | This column reflects the average of the Compensation Actually Paid for our NEOs as a group (excluding our CEO or CEOs, in the case of 2020) for each of the periods presented. The amounts in the following table reflect the adjustments to the Summary Compensation Table Total to determine Compensation Actually Paid. The NEOs included for purposes of computing the amounts in this column are listed in footnote (5) above. Adjustments (additions/deductions) are not listed for Pension/Non-Qualified Deferred Compensation because we do not offer these plans. |
| Year | Summary Compensation Table Total | Amounts Deducted from Grant Date Value of Annual Equity | Amounts Added for the Fair Value of Awards Granted During Fiscal Year | Amounts Added for Awards Granted & Vested During Fiscal Year | Amounts Add/ Deducted for Awards that Vested During Fiscal Year | Amounts Added/ Deducted for the Change in Fair Value of Awards Outstanding at Fiscal Year End(a) | Compensation Actually Paid | |||||||||||||||
| 2023 | $ | 7,376,771 | $ | (5,590,153) | $ | 8,606,944 | $ | 812,533 | $ | 236,286 | $ | 6,661,784 | $ | 18,104,165 | ||||||||
| 2022 | $ | 8,982,257 | $ | (7,261,199) | $ | 9,489,852 | $ | 1,062,531 | $ | 538,093 | $ | 5,451,796 | $ | 18,263,330 | ||||||||
| 2021 | $ | 7,193,835 | $ | (5,687,759) | $ | 6,319,320 | $ | 1,125,037 | $ | (778,741) | $ | 797,359 | $ | 8,969,051 | ||||||||
| 2020 | $ | 6,697,408 | $ | (4,812,998) | $ | 6,038,487 | $ | — | $ | 1,048,920 | $ | 1,612,567 | $ | 10,584,384 | ||||||||
| 2019 | $ | 4,668,405 | $ | (3,093,700) | $ | 4,011,590 | $ | 103,204 | $ | 866,837 | $ | 1,272,139 | $ | 7,828,475 | ||||||||
| (a) | Pursuant to applicable SEC rules, the fair values at the end of each fiscal year for the financial PSU awards were valued at 200% as of December 31, 2023, 2022, 2021, 2020, 2019, and 2018; and, the non-financial PSU award for 2016 was valued at 200% as of December 31, 2018; the non-financial PSU award for 2017 was valued at 200% as of December 31, 2019 and 2018; the non-financial PSU award for 2018 was valued at 200%, 100% and 100% as of December 31, 2020, 2019 and 2018, respectively; the non-financial PSU award for 2019 was valued at 200%, 100% and 50% as of December 31, 2021, 2020 and 2019; the non-financial PSU award for 2020 was valued at 200%, 50% and 50% as of December 31, 2022, 2021 and 2020; the non-financial PSU award for 2021 was valued at 100%, 50% and 50% as of December 31, 2023, 2022 and 2021, respectively; the non-financial PSU award for 2022 was valued at 50% and 0% as of December 31, 2023 and 2022, respectively; the non-financial PSU award for 2023 was valued at 50% as of December 31, 2023. |
| (7) | This column represents our cumulative total shareholder return (“TSR”) under SEC rules from December 31, 2018, the last trading day before the start of 2019, through the last trading day for the applicable fiscal year in the table. TSR is calculated by dividing the sum of the cumulative amount of dividends for the measurement period, assuming dividend reinvestment, and the difference between our share price at the end and the beginning of the measurement period by our share price at the beginning of the measurement period. |
| (8) | This column represents the TSR of the NBI, our selected peer group, which is the same peer group used for purposes of Item 201(e) of Regulation S-K from December 31, 2018, the last trading day before the start of 2019, through the last trading day for the applicable fiscal year in the table, assuming reinvestment of dividends and weighted according to the respective companies’ stock market capitalization at the beginning of each period for which a return is indicated. |
| (9) | This column reflects net income as reported for each year in our Annual Report on Form 10-K. |
| (10) | This column reflects net product revenue as reported for each year in our Annual Report on Form 10-K. For purposes of the table above, we have selected net product revenue as the financial performance measure representing the most important financial performance measure used to link executive compensation actually paid to our financial performance in the most recently completed fiscal year. Please the Compensation Discussion and Analysis section beginning on page 50 of this proxy statement for additional information. |
The relationships between certain specified measures in the sole discretion of our board, after written notice of any such act or failure to act and a reasonable opportunity to curepay-versus-performance table over the deficiency has beenfive most recently completed fiscal years are provided to Dr. Chodakewitz, to be (A) willful gross neglect or (B) willful gross misconduct, resulting, in either case, in material harm to us unless such act, or failure to act, was believed by him, in good faith, to be in our best interests.
| (1) | 2020 Compensation Actually Paid represented here reflects 2020 compensation of Reshma Kewalramani, our current CEO, and excludes 2020 compensation paid to Jeffrey Leiden, our former CEO. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 86
| (1) | ||
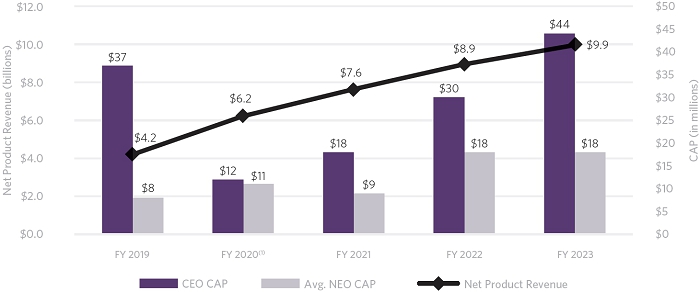
| (1) | 2020 Compensation Actually Paid represented here reflects 2020 compensation of Reshma Kewalramani, our current CEO, and excludes 2020 compensation paid to Jeffrey Leiden, our former CEO. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 83
The tabular list below includes the three financial and non-financial performance measures that represent the most important performance measures used to link compensation actually paid to company performance in 2023. The performance measures included in this list are not ranked by relative importance.
| Performance Measures Used to Link Executive Compensation Actually Paid to Company Performance for the Most Recently Completed Fiscal Year | ||
| Net Product Revenue | ||
| Adjusted Non-GAAP EBITDA(2) |
| Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options | Weighted-Average Exercise Price of Outstanding Options | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in first column) | ||
| Equity Compensation Plans Approved by Shareholders (1) | 11,145,334 | $75.99 | 15,288,603 | ||
| Equity Compensation Plans Not Approved by Shareholders | — | — | — | ||
| Total | 11,145,334 | 15,288,603 | |||
| (1) | |
| (2) | Adjusted non-GAAP EBITDA is a financial performance measure used to link compensation actually paid to company financial performance, as described in Goals – Financial Strength. We calculated adjusted non-GAAP EBITDA by excluding interest, taxes, depreciation and amortization expenses, as well as AIPR&D, from net income, as well as excluding each of our |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 85
| Back of Contents |
The following table sets forth information regarding beneficial ownership of our common stock as of
| Name and Address | Shares Beneficially Owned (1) | Percentage of Total (2) | ||
| T. Rowe Price Associates, Inc. (3) | 25,345,121 | 10.2 | % | |
| 100 E. Pratt Street | ||||
| Baltimore, Maryland 21202 | ||||
| Capital World Investors (4) | 20,737,911 | 8.4 | % | |
| 333 South Hope Street | ||||
| Los Angeles, California 90071 | ||||
| BlackRock, Inc. (5) | 18,229,864 | 7.4 | % | |
| 55 East 52nd Street | ||||
| New York, New York 10055 | ||||
| FMR LLC (6) | 15,009,184 | 6.1 | % | |
| 245 Summer Street | ||||
| Boston, Massachusetts 02210 | ||||
| Wellington Management Group LLP (7) | 14,863,986 | 6.0 | % | |
| 280 Congress Street | ||||
| Boston, Massachusetts 02210 | ||||
| The Vanguard Group (8) | 14,503,469 | 5.9 | % | |
| 100 Vanguard Blvd. | ||||
| Malvern, Pennsylvania 19355 | ||||
| JPMorgan Chase & Co. (9) | 12,630,441 | 5.1 | % | |
| 270 Park Ave. | ||||
| New York, New York 10017 | ||||
| Sangeeta N. Bhatia (10) | 7,500 | * | ||
| Joshua Boger (10) | 1,374,970 | * | ||
| Terrence C. Kearney (10) | 61,875 | * | ||
| Yuchun Lee (10) | 82,500 | * | ||
| Jeffrey M. Leiden (10) | 1,326,254 | * | ||
| Margaret G. McGlynn (10) | 81,088 | * | ||
| Bruce I. Sachs (10) | 141,699 | * | ||
| Elaine S. Ullian (10) | 72,765 | * | ||
| William D. Young (10) | 55,000 | * | ||
| Ian F. Smith (10) | 406,377 | * | ||
| David Altshuler (10) | 207,586 | * | ||
| Stuart A. Arbuckle (10) | 310,897 | * | ||
| Jeffrey Chodakewitz (10) | 254,647 | * | ||
| All directors and executive officers as a group (16 persons) (10) | 4,797,821 | 1.9 | % | |
 | each shareholder known by us to be the beneficial owner of more than 5% of our common stock on that date; |
 | each of our directors and our director nominee; |
 | each NEO; and |
 | all directors and executive officers as a group. |
| Name and Address | Shares Beneficially Owned(1) | Percentage of Total(2) | |
| The Vanguard Group(3) | 22,281,729 | 8.6% | |
| 100 Vanguard Blvd. | |||
| Malvern, Pennsylvania 19355 | |||
| Capital World Investors(4) | 22,080,207 | 8.5% | |
| 333 South Hope Street, 55th Floor | |||
| Los Angeles, California 90071 | |||
| BlackRock, Inc.(5) | 21,881,203 | 8.5% | |
| 50 Hudson Yards | |||
| New York, New York 10001 | |||
| FMR LLC(6) | 12,981,286 | 5.0% | |
| 245 Summer Street | |||
| Boston, Massachusetts 02210 | |||
| Sangeeta N. Bhatia(7) | 5,839 | * | |
| Lloyd Carney(7) | 5,318 | * | |
| Alan Garber(7) | 32,887 | * | |
| Terrence C. Kearney(7) | 41,366 | * | |
| Reshma Kewalramani(7) | 62,369 | * | |
| Michel Lagarde(7) | — | * | |
| Jeffrey M. Leiden(7) | 151,292 | * | |
| Diana McKenzie(7) | 7,632 | * | |
| Bruce I. Sachs(7) | 95,537 | * | |
| Jennifer Schneider(7) | — | * | |
| Nancy Thornberry(7) | — | * | |
| Suketu Upadhyay(7) | 2,961 | * | |
| David M. Altshuler(7) | — | * | |
| Stuart A. Arbuckle(7) | 3,932 | * | |
| Charles F. Wagner, Jr.(7) | 36,570 | * | |
| All directors and executive officers as a group (20 persons)(7) | 597,104 | 0.2% |
| * | Less than 1%
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 89
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 90
OTHER | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 | the name and address of the shareholder who intends to make the nomination and of the person or persons to be nominated; |
 | a representation that the shareholder is a holder of record of our stock entitled to vote at such meeting and intends to appear personally or by proxy at the meeting to nominate the person or persons specified in the notice; |
 | a description of all arrangements or understandings between the shareholder and each nominee and any other person or persons (naming such person or persons) pursuant to which the nomination or nominations are to be made by the shareholder; |
 | the other information regarding each nominee proposed by the shareholder that would be required to be included in a proxy statement filed pursuant to the proxy rules of the SEC; and |
 | the consent of each nominee to serve on our board of directors if so elected. |
If a shareholder wishes to nominate a candidate for election to our board of directors at the 20172025 annual meeting of shareholders, and is eligible and elects to have such candidate included in the proxy statement for our 20172025 annual meeting of shareholders pursuant to our proxy access by-law, such nomination maymust be submitted to our corporate secretary no earlier than January 15, 2025 and no later than February 14, 2025, and must include, in addition to the information set forth above for other shareholder nominees, the information set forth in Sections 13 and 14 of Article I and Section 8 of Article II of our by-laws.
If a shareholder wishes to solicit proxies for a shareholder nominee for election to our board at the 2024 annual meeting of shareholders pursuant to Rule 14a-19 of the Exchange Act, notice must be submitted to our corporate secretary no later than March 17, 201716, 2025. Such solicitation and notice must include,comply with the requirements of Rule 14a-19 of the Exchange Act and our by-laws.
Discretionary Voting Authority. If we do not receive notice of a matter to be considered for presentation at the 2025 annual meeting of shareholders by the dates specified in additionour advanced notice provisions applicable to such matter (or, in the absence of such a provision, by February 19, 2025), our proxy holders will have the right to exercise discretionary voting authority with respect to such matter without including such matter in our proxy materials.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 91
Any shareholder who wishes to address questions regarding our business directly with our board of directors, or any individual director,director(s), should direct his or hersuch questions, in writing, in care of our corporate secretary, to our offices at 50 Northern Avenue, Boston, Massachusetts 02210. Under procedures approved by our board, including a majority of our independent directors, allAll substantive communications shall be reviewed by our corporate secretary and forwarded or reported to the chair of the corporate governance and nominating committee,CGNC, the lead independent directorsdirector and/or our full board, as deemed appropriate, with the exception of those communications relating to ordinary or routine business affairs, personal grievances, or matters as to which we tend to receive repetitive or duplicative communications.
Some banks, brokers and other nominee record holders may be participating in the practice of “householding” proxy statements and annual reports.materials. This means that only one copy of our Notice of Internet Availability of Proxy Materials or proxy statement and annual report may have been sent to multiple shareholders in your household. We will promptly deliver a separate copy of these documents to you if you write or call our corporate secretary at the following address or phone number: 50 Northern Avenue, Boston, Massachusetts 02210, telephone (617) 341-6100. If you want to receive separate copies of the annual report and proxy statementmaterials in the future, or if you are receiving multiple copies and would like to receive only one copy for your household, you should contact your bank, broker, or other nominee
We will bear the cost of soliciting proxies, including expenses in connection with preparing and mailing this proxy statement.statement and hosting the annual meeting. We have retained MacKenzie Partners, Inc.Morrow Sodali to assist in the solicitation of proxies at an estimated cost of approximately $20,000. Proxies also may be solicited by our directors and employees by mail, by telephone, in person or otherwise. EmployeesNeither directors nor employees will not receive additional compensation for solicitation efforts. In addition, we will request banks, brokers and other custodians, nominees and fiduciaries to forward proxy material to the beneficial owners of common stock and to obtain voting instructions from the beneficial owners. We will reimburse those firms for their reasonable expenses in forwarding proxy materials and obtaining voting instructions.
This proxy statement contains forward-looking statements. Forward-looking statements are not purely historical and maybe accompanied by words such as defined in the Private Securities Litigation Reform Act“anticipates,” “may,” “forecasts,” “expects,” “intends,” “plans,” “potentially,” “believes,” “seeks,” “estimates,” and other words and terms of 1995, including,similar meaning. Such statements may related to, without limitation, statements regarding our medicines, statementsmarketed products and pipeline therapies, particularly with respect to benefits and safety, potential regulatory approval of our drug candidatessubmissions and approvals, expected clinical development plans and timing, and expectations regarding commercialization of certain pipeline therapies, the data that will be generated by ongoing and planned clinical trials and the ability to use that data to advance compounds, continue development or support regulatory filings, including the durable efficacy and effectiveness of CASGEVY as a potential one-time functional cure for people with severe SCD and TDT, our beliefs and plans with respect to the potential near-term launch of our vanzacaftor triple for the treatment of CF and for VX-548 for the treatment of acute pain, as well as statements with respect to Vertex'sVertex’s potential future financial performance.performance and our beliefs regarding the patient populations for CF, SCD, TDT and pain, and those potentially eligible for our therapies. While we believe the forward-looking statements contained in this proxy statement are accurate, these forward-looking statements represent the our beliefs only as of the date of this proxy statement and therestatement. There are a number of factorsrisks and uncertainties that are difficult to predict and could cause actual events or results to differ materially from those indicated by such forward-looking statements, includingstatements. Those risks and uncertainties include, among other things, that the company’s expectations regarding its future financial performance may be incorrect (including because one or more of the company’s assumptions underlying its expectations may not be realized), that data from preclinical testing or clinical trials, especially if based on a limited number of patients, may not be indicative of final results, that regulatory authorities may not approve regulatory filings for our pipeline products on a timely basis, or at all, that data from the company’s developmental programs may not support registration or further development programs may not support registration or further development of its potential medicines in a timely manner, or at all, due to safety, efficacy or other reasons, and other risks listed under Risk Factorsthe heading “Risk Factors” in our Annual Report on Form 10-K for the fiscal year ended
Website references are provided throughout this document for convenience. The content on the referenced website does not constitute part of and is not incorporated by reference into this proxy statement.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 92
As of February 29, 2024, there were 14,519,973 shares remaining available for award under our Amended and Restated 2013 Stock and Option Plan (our “2013 Plan”). Under our 2013 Plan, all awards may be granted as full value awards but count as 1.66 shares for each full value share awarded.
As of February 29, 2024, under our equity plans:
 | Stock options covering 1,835,575 shares of our common stock, with a weighted average exercise price of $152.28 and a weighted average remaining term of 3.85 years, were outstanding; and |
 | Unvested PSUs and RSUs covering 4,004,859 shares of our common stock were outstanding. |
The following table provides aggregate information with respect to all of our equity compensation plans in effect as of December 31, 2023. We are required under applicable SEC rules to disclose in this table the number of shares remaining available for issuance under our equity plans as of December 31, 2023. Accordingly, the figures in the table below do not reflect the equity grants made to our employees under the 2013 Plan, since December 31, 2023.
| Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Restricted Stock Units and Rights | Weighted-Average Exercise Price of Outstanding Options and Rights | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (excluding securities reflected in first column) | ||||
| Equity Compensation Plans Approved by Shareholders(1) | 6,097,101(2) | $ 151.37(3) | 17,821,975 | (4) | |||
| Equity Compensation Plans Not Approved by Shareholders | — | — | — | ||||
| TOTAL | 6,097,101 | 17,821,975 | |||||
| (1) | These plans consist of the 2013 Plan, the Amended and Restated 2006 Stock and Option Plan (the “2006 Plan”) and our Employee Stock Purchase Plan. No further shares of common stock will be issued or distributed under the 2006 Plan. |
| (2) | Represents the number of underlying shares of common stock associated with outstanding options, RSUs, PSUs, and deferred stock units granted under shareholder approved plans, as of December 31, 2023, and includes 1,897,112 options granted under the 2013 Plan, 2,941,980 RSUs granted under the 2013 Plan, 1,195,427 PSUs (assuming the maximum number of PSUs will be earned) granted under the 2013 Plan, 19,624 deferred stock units attributable to compensation deferred by non-employee directors participating in the Director Plan and distributable in the form of shares of common stock under the 2013 Plan (and which are treated as outstanding “stock rights” under the 2013 Plan), and 42,958 options granted under the 2006 Plan. |
| (3) | Represents the weighted-average exercise price of options outstanding under the 2013 Plan and 2006 Plan. See note (2) above with respect to RSUs, PSUs and deferred stock units (credited under the Director Plan) outstanding under the 2013 Plan. The weighted-average exercise price does not take these awards into account. |
| (4) | Represents the number of shares available for future issuance under shareholder approved equity compensation plans and consists of 16,508,935 shares available for future issuance under the 2013 Plan and 1,313,040 shares available for future issuance under the Employee Stock Purchase Plan, including shares to be purchased at the end of the current offering period ending May 15, 2024. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 93
At the annual meeting, shareholders will act upon the matters outlined in the Notice of Annual Meeting of Shareholders. These include:
 | The election of directors; |
 | The ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm; |
 | To hold an advisory vote on our 2023 named executive officer compensation; |
 | A shareholder proposal regarding special shareholder meeting improvement, if properly presented at the meeting; and |
 | A shareholder proposal regarding a report on racial and gender pay gaps, if properly presented at the meeting. |
Management, members of our board and representatives of Ernst & Young LLP are expected to attend the annual meeting and be available to respond to appropriate questions from shareholders.
It is your legal designation of another person to vote the stock you own in the manner you direct. That other person is called a proxy. If you designate someone as your proxy in a written document, that document also is called a proxy or a proxy card. The board of directors has designated Jeffrey Leiden, Reshma Kewalramani, Jonathan Biller, and Joy Liu to serve as proxies at the annual meeting.
It is a document that provides certain information about a company and matters to be voted upon at a meeting of shareholders. The rules of the SEC and other applicable laws require us to give you, as a shareholder, the information in this proxy statement and our Annual Report when we are soliciting your vote.
We are distributing our proxy materials to shareholders via the Internet under the “Notice and Access” approach permitted by rules of the SEC. This approach provides a timely and convenient method of accessing the materials and voting. On or about April 4, 2024, we will begin mailing a “Notice of Internet Availability of Proxy Materials” to shareholders. This notice includes instructions on how to access our notice of annual meeting of shareholders, this proxy statement and our Annual Report on Form 10-K for the year ended December 31, 2023 and how to vote your shares. The Notice of Internet Availability of Proxy Materials also contains instructions on how to receive a paper copy of the proxy materials and our Annual Report, if you prefer.
Shareholders of Record. If your shares are registered in your name with our transfer agent, Computershare, you are a shareholder of record with respect to those shares, and 2016the Notice of Internet Availability of Proxy Materials was sent directly to you by Computershare.
Street Name Holders. If you hold your shares in an account at a bank, broker or other nominee, then you are the beneficial owner of shares held in “street name.” The Notice of Internet Availability of Proxy Materials was forwarded to you by your bank, broker or other nominee. As a beneficial owner, you have the right to direct your bank or broker how to vote the shares held in your account.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement | 89
We will hold a virtual annual meeting this year. The virtual meeting will facilitate shareholder attendance and participation by enabling all shareholders to attend and participate in the annual meeting from any location and at no cost. Visit https://meetnow.global/MQ4XFU9 to attend and submit questions during the meeting. No physical meeting will be held this year. To attend the virtual meeting, shareholders of record will not need to register in advance but will need the control number included on their Notice of Internet Availability of Proxy Materials or proxy card, or within the body of the email sending the proxy statement. Shareholders whose shares are held in street name may attend the annual meeting by registering and obtaining a control number in advance using the instructions below under the heading “How do I Register to Attend the Virtual Annual Meeting on the Internet?” The control number will be required to attend and participate in the virtual annual meeting, including voting your shares electronically and submitting questions.
If you would like to submit a question related to the business of the meeting, you may do so during the meeting by logging into the virtual annual meeting website and entering the control number included on your Notice of Internet Availability of Proxy Materials, proxy card, voting instruction form or electronic notification when prompted.
The meeting webcast will begin promptly at 9:00 a.m. (Eastern Time). We encourage you to access the meeting prior to the start time. You should allow for ample time for the check-in procedures.
If you are a registered shareholder (i.e., you hold your shares through our transfer agent, Computershare), you do not need to register in advance to attend the virtual annual meeting. Please follow the instructions on the Notice of Internet Availability of Proxy Materials or the proxy card that you received.
If you hold your shares in street name through an intermediary, such as a bank or broker, you must register and obtain a control number in advance to attend the annual meeting on the Internet.
To register to attend the virtual annual meeting you will need to obtain a legal proxy from your bank, broker or other nominee. Once you have received a legal proxy from them, you must send an email attaching an image of your legal proxy from your bank, broker or other nominee to legalproxy@computershare.com, along with your name and email address. Alternatively, you may mail your legal proxy to the following address: Computershare, Vertex Pharmaceuticals Incorporated Legal Proxy, P.O. Box 43001, Providence, RI 02940-3001. Requests for registration must be labeled as “Legal Proxy” and be received no later than 5:00 p.m (Eastern Time) on May 10, 2024. After Computershare receives your registration materials, you will receive an email from Computershare confirming your registration and providing your control number which will allow you to fully participate in the annual meeting virtually.
The virtual meeting platform is fully supported across browsers (MS Edge, Firefox, Chrome and Safari) and devices (desktops, laptops, tablets and cell phones) running the most up-to-date version of applicable software and plugins. Please note that Internet Explorer is not a supported browser. You should ensure that you have a strong WiFi connection wherever you intend to participate in the meeting. We encourage you to access the meeting prior to the start time. For further assistance should you need it you may call 1-888-724-2416.
In order for us to conduct the annual meeting, holders of a majority of the shares entitled to vote as of the close of business on the record date must be present in person or by proxy. This constitutes a quorum. Shares present virtually during the annual meeting will be considered shares of common stock represented in person at the meeting. If you are a shareholder of record, your shares are counted as present if you properly vote by Internet, telephone, return a proxy card by mail or if you attend the annual meeting online. If you are the beneficial owner of shares held in street name, you must follow the instructions of your bank or broker in order to direct them how to vote the shares held in your account or obtain a legal proxy to vote online at the annual meeting. Abstentions and broker non-votes will be counted as present for purposes of establishing a quorum. If a quorum is not present, we will adjourn the annual meeting until a quorum is obtained.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 95
If you are a shareholder of record, you may vote your shares by one of the following methods:
| 1. | Vote by Internet by going to the web address www.envisionreports.com/VRTX before the annual meeting and following the instructions for Internet voting on the Notice of Internet Availability or proxy card. Have the Notice of Internet Availability of Proxy Materials, which contains your control number, available when voting by Internet. |
| 2. | Vote by proxy card, if you have received written proxy materials, by completing, signing, dating, and mailing your proxy card in the envelope provided. If you vote by Internet, please do not mail your proxy card. |
| 3. | Vote by telephone by following the instructions on the Notice of Internet Availability of Proxy Materials or proxy card. |
| 4. | By attending the annual meeting online. During the annual meeting, you may vote online by following the instructions at https://meetnow.global/MQ4XFU9. Have the Notice of Internet Availability of Proxy Materials, which contains your control number, available when you access the virtual meeting webpage. |
If you are a street name holder, your bank, broker or other nominee will provide you with a form seeking instruction on how your shares should be voted.
The record date for the annual meeting is March 18, 2024 and was established by our board of directors. On the record date, there were 258,459,343 shares of our common stock outstanding, each of which is entitled to one vote on each matter properly brought before the annual meeting. Owners of record of common stock at the close of business on the record date are entitled to:
 | receive notice of the annual meeting; and |
 | vote at the annual meeting and any adjournment or postponement of the annual meeting. |
Shareholders may revoke a proxy and/or change their vote prior to the completion of voting at the annual meeting by:
 | subsequently submitting a vote by Internet at www.envisionreports.com/VRTX or by telephone by following the directions on the Notice of Internet Availability of Proxy Materials, voting instruction form or your proxy card; |
 | signing another proxy card with a later date and delivering it to our corporate secretary at 50 Northern Avenue, Boston, Massachusetts 02210, before the annual meeting; or |
 | voting at the annual meeting online, if you are a shareholder of record or hold your shares in street name and have obtained a legal proxy from your bank or broker. |
Shareholders should specify their choice for each matter following the directions described on their Notice of Internet Availability of Proxy Materials or proxy card. If no specific instructions are given, proxies that are signed and returned will be voted:
 | FOR the election of each director nominee; |
 | FOR ratification of the appointment of Ernst & Young LLP as our independent registered public accounting firm for the year ending December 31, 2024; |
 | FOR our 2023 named executive officer compensation; |
 | AGAINST the shareholder proposal regarding special shareholder meeting improvement; and |
 | AGAINST the shareholder proposal regarding a report on racial and gender pay gaps. |
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 96
If you are a shareholder of record and do not provide a proxy, you must attend the annual meeting in order to vote. If you hold shares through an account with a bank or broker, your shares may be voted by the bank or broker on certain matters if you do not provide voting instructions. Banks and brokers have the authority under applicable rules to vote shares on routine matters for which their customers do not provide voting instructions. The ratification of Ernst & Young LLP as our independent registered public accounting firm is considered a routine matter. Each of the other proposals, including the election of directors, the advisory vote with respect to our executive compensation program, and the two shareholder proposals are considered not routine, and banks and brokers cannot vote shares without instruction on those matters. Shares that banks and brokers are not authorized to vote on those matters are counted as “broker non-votes” and will have no effect on the results of those votes.
To be elected, the number of votes cast “FOR” each director nominee must exceed the number of votes cast “AGAINST” that nominee. Abstentions will have no effect on the results of this vote. Our Corporate Governance Principles contain procedures to be followed in the event that one or more directors do not receive a majority of the votes cast “FOR” their election.
To be approved, this proposal must receive an affirmative vote from shareholders present personally or represented by proxy at the annual meeting representing a majority of the votes cast on the proposal. Abstentions will have no effect on the results of this vote.
To be approved, this proposal must receive an affirmative vote from shareholders present personally or represented by proxy at the annual meeting representing a majority of the votes cast on the proposal. Abstentions will have no effect on the results of this vote.
To be approved, this proposal must receive an affirmative vote from shareholders present personally or represented by proxy at the annual meeting representing a majority of the votes cast on the proposal. Abstentions will have no effect on the results of this vote.
To be approved, this proposal must receive an affirmative vote from shareholders present personally or represented by proxy at the annual meeting representing a majority of the votes cast on the proposal. Abstentions will have no effect on the results of this vote.
The SEC has an informational website that provides shareholders with general information about how to cast their vote and why voting should be an important consideration for shareholders. You may access that website at sec.gov/spotlight/proxymatters.shtml.
VERTEX PHARMACEUTICALS INCORPORATED - 2024 Proxy Statement 97


